Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
- PMID: 37482655
- PMCID: PMC10555535
- DOI: 10.4093/dmj.2023.0067
Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Abstract
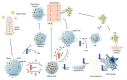
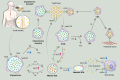
Dyslipidemia is a potentially modifiable cardiovascular risk factor. Whereas the recommendations for the treatment target of dyslipidemia in the general population are being more and more rigorous, the 2013 Kidney Disease: Improving Global Outcomes clinical practice guideline for lipid management in chronic kidney disease (CKD) presented a relatively conservative approach with respect to the indication of lipid lowering therapy and therapeutic monitoring among the patients with CKD. This may be largely attributed to the lack of high-quality evidence derived from CKD population, among whom the overall feature of dyslipidemia is considerably distinctive to that of general population. In this review article, we cover the characteristic features of dyslipidemia and impact of dyslipidemia on cardiovascular outcomes in patients with CKD. We also review the current evidence on lipid lowering therapy to modify the risk of cardiovascular events in this population. We finally discuss the association between dyslipidemia and CKD progression and the potential strategy to delay the progression of CKD in relation to lipid lowering therapy.
Keywords: Dyslipidemias; Kidney diseases; Risk.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord. 2017;18:29–40. - PubMed
-
- Mesquita J, Varela A, Medina JL. Dyslipidemia in renal disease: causes, consequences and treatment. Endocrinol Nutr. 2010;57:440–8. - PubMed
-
- Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

