Immunological mechanisms underlying clinical phenotypes and noninvasive diagnosis of immune checkpoint inhibitor-induced kidney disease
- PMID: 37482912
- PMCID: PMC10865966
- DOI: 10.1111/imr.13243
Immunological mechanisms underlying clinical phenotypes and noninvasive diagnosis of immune checkpoint inhibitor-induced kidney disease
Abstract
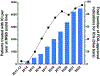
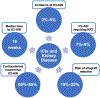
Immune checkpoint inhibitors (ICIs) have become a mainstay of cancer therapy, with over 80 FDA-approved indications. Used in a variety of settings and in combination with each other and with traditional chemotherapies, the hyperactive immune response induced by ICIs can often lead to immune-related adverse events in bystander normal tissues such as the kidneys, lungs, and the heart. In the kidneys, this immune-related adverse event manifests as acute interstitial nephritis (ICI-AIN). In the era of widespread ICI use, it becomes vital to understand the clinical manifestations of ICI-AIN and the importance of prompt diagnosis and management of these complications. In this review, we delve into the clinical phenotypes of ICI-AIN and how they differ from traditional drug-induced AIN. We also detail what is known about the mechanistic underpinnings of ICI-AIN and the important diagnostic and therapeutic implications behind harnessing those mechanisms to further our understanding of these events and to formulate effective treatment plans to manage ICI-AIN.
Keywords: Th1/Th2/Th17 cells; acute interstitial nephritis; biomarkers; immune-mediated diseases; immunotherapies.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Nigro O, Pinotti G, de Galitiis F, et al. Late immune-related adverse events in long-term responders to PD-1/PD-L1 checkpoint inhibitors: a multicentre study. Eur J Cancer. 2020;134:19–28. - PubMed
-
- Schulz TU, Zierold S, Sachse MM, et al. Persistent immune-related adverse events after cessation of checkpoint inhibitor therapy: prevalence and impact on patients’ health-related quality of life. Eur J Cancer. 2022;176:88–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources