An HGF-dependent positive feedback loop between bladder cancer cells and fibroblasts mediates lymphangiogenesis and lymphatic metastasis
- PMID: 37483113
- PMCID: PMC10693311
- DOI: 10.1002/cac2.12470
An HGF-dependent positive feedback loop between bladder cancer cells and fibroblasts mediates lymphangiogenesis and lymphatic metastasis
Abstract
Background: Cancer-associated fibroblasts (CAFs) play a vital role in facilitating tumor progression through extensive reciprocal interplay with cancer cells. Tumor-derived extracellular vesicles (EVs) are the critical mediators involved in the crosstalk between cancer cells and stromal cells, contributing to the metastasis of cancers. Yet, the biological mechanisms of tumor-derived EVs in triggering CAFs phenotype to stimulate the lymph node (LN) metastasis of bladder cancer (BCa) are largely unknown. Here, we aimed to explore the effects and molecular mechanisms of tumor-derived EV-mediated CAFs phenotype in regulating BCa LN metastasis.
Methods: The high-throughput sequencing was utilized to identify the crucial long non-coding RNA (lncRNA) associated with CAF enrichment in BCa. The functional role of the transition of fibroblasts to CAFs induced by LINC00665-mediated EVs was investigated through the in vitro and in vivo assays. Chromatin isolation by RNA purification assays, fluorescence resonance energy transfer assays, cytokine profiling and patient-derived xenograft (PDX) model were performed to explore the underlying mechanism of LINC00665 in the LN metastasis of BCa.
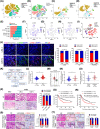
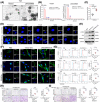
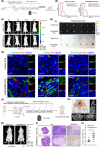
Results: We found that CAFs are widely enriched in the tumor microenvironment of BCa, which correlated with BCa lymphangiogenesis and LN metastasis. We then identified a CAF-associated long non-coding RNA, LINC00665, which acted as a crucial mediator of CAF infiltration in BCa. Clinically, LINC00665 was associated with LN metastasis and poor prognosis in patients with BCa. Mechanistically, LINC00665 transcriptionally upregulated RAB27B expression and induced H3K4me3 modification on the promoter of RAB27B through the recruitment of hnRNPL. Moreover, RAB27B-induced EVs secretion endowed fibroblasts with the CAF phenotype, which reciprocally induced LINC00665 overexpression to form a RAB27B-HGF-c-Myc positive feedback loop, enhancing the lymphangiogenesis and LN metastasis of BCa. Importantly, we demonstrated that blocking EV-transmitted LINC00665 or HGF broke this loop and impaired BCa lymphangiogenesis in a PDX model.
Conclusion: Our study uncovers a precise mechanism that LINC00665 sustains BCa LN metastasis by inducing a RAB27B-HGF-c-Myc positive feedback loop between BCa cells and fibroblasts, suggesting that LINC00665 could be a promising therapeutic target for patients with LN metastatic BCa.
Keywords: Cancer-associated fibroblasts; HGF; bladder cancer; extracellular vesicles; long non-coding RNA; lymph node metastasis; lymphangiogenesis; positive feedback loop.
© 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur Urol. 2014;66(6):1065–77. - PubMed
-
- Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324(19):1980–91. - PubMed
-
- Cathomas R, Lorch A, Bruins HM, Comperat EM, Cowan NC, Efstathiou JA, et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur Urol. 2022;81(1):95–103. - PubMed
-
- Escobedo N, Oliver G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu Rev Cell Dev Biol. 2016;32:677–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFA1305500/National Key Research and Development Program of China
- 2018YFA0902803/National Key Research and Development Program of China
- 82173272/National Natural Science Foundation of China
- 82173271/National Natural Science Foundation of China
- 81825016/National Natural Science Foundation of China
- 82103536/National Natural Science Foundation of China
- 82103416/National Natural Science Foundation of China
- 81871945/National Natural Science Foundation of China
- 81902589/National Natural Science Foundation of China
- 2021B1515020091/Guangdong Basic and Applied Basic Research Foundation
- 2020A1515010815/Guangdong Basic and Applied Basic Research Foundation
- 2018B010109006/Guangdong Basic and Applied Basic Research Foundation
- 2021A1515010355/Guangdong Basic and Applied Basic Research Foundation
- 202002030388/Science and Technology Program of Guangzhou, China
- 201803010049/Science and Technology Program of Guangzhou, China
- 2017B020227007/Science and Technology Program of Guangzhou, China
LinkOut - more resources
Full Text Sources
Medical

