Layered Double Hydroxides for Regulating Phosphate in Water to Achieve Long-Term Nutritional Management
- PMID: 37483187
- PMCID: PMC10357453
- DOI: 10.1021/acsomega.3c02576
Layered Double Hydroxides for Regulating Phosphate in Water to Achieve Long-Term Nutritional Management
Abstract
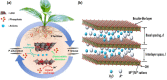
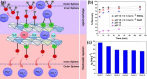
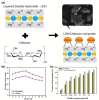
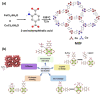
Hunger and undernourishment are increasing global challenges as the world's population continuously grows. Consequently, boosting productivity must be implemented to reach the global population's food demand and avoid deforestation. The current promising agricultural practice without herbicides and pesticides is fertilizer management, particularly that of phosphorus fertilizers. Layered double hydroxides (LDHs) have recently emerged as favorable materials in phosphate removal, with practical application possibilities in nanofertilizers. This review discusses the fundamental aspects of phosphate removal/recycling mechanisms and highlights the current endeavors on the development of phosphate-selective sorbents using LDH-based materials. Specific emphasis is provided on the progress in designing LDHs as the slow release of phosphate fertilizers reveals their relevance in making agro-practices more ecologically sound. Relevant pioneering efforts have been briefly reviewed, along with a discussion of perspectives on the potential of LDHs as green nanomaterials to improve food productivity with low eco-impacts.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources

