Rapid Analytical Method Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Exemestane and Genistein with Specific Application in Lipid-Based Nanoformulations
- PMID: 37483215
- PMCID: PMC10357584
- DOI: 10.1021/acsomega.3c01791
Rapid Analytical Method Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Exemestane and Genistein with Specific Application in Lipid-Based Nanoformulations
Abstract
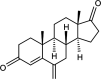
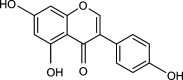
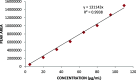
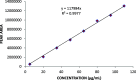
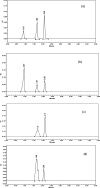
Exemestane (EXE), an irreversible aromatase inhibitor, is employed as a therapy for hormone-dependent breast cancer. Several studies have also established the budding effects of genistein (GEN) in various types of cancer such as breast, prostate, as well as skin due to its feeble estrogenic and anti-estrogenic properties. Considering the promising benefits of GEN, it was combined with EXE to accomplish superior therapeutic efficiency with fewer side effects. The quantification of the exact concentration of EXE and GEN when delivered as a combination would be required for which HPLC method was developed and validated. For this purpose, the C18 ODS column having dimensions of 150 × 4.6 mm, 5 μm, using mobile phase A as methanol:water (35:15, v/v), with formic acid (0.01%), and B as acetonitrile (in the ratio of A:B--30:70 v/v) at a flow rate of 1 mL/min was commonly used. The Box-Behnken design was chosen as our experimental model, and the interactions among the independent and dependent variables were analyzed. Parameters like linearity, system suitability, specificity, precision (intra- and interday), robustness, ruggedness, LOD (limit of detection), and LOQ (limit of quantification) were selected for the validation of our proposed method. EXE and GEN were eluted individually at 245 and 270.5 nm, respectively, while both of the agents were determined simultaneously at 256 nm, showing retention time as 2.10 and 1.67 min, respectively, and the calibration plot was observed to be linear in the range of 5-110 μg/mL. Hence, the method that we developed and validated was found to be suitable for the identification of both the drugs simultaneously in combination and in our in-house-developed nanoformulation.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Fein L.; Per B.; Lønning E.; Bajetta E.; Murray R.; Eisenberg P. D.; Mickiewicz E.; Celio L.; Pitt P.; Mita M.; Aaronson N. K.; Fowst C.; Arkhipov A.; Salle E.; Polli A.; Massimini G.. Activity of Exemestane in Metastatic Breast Cancer a Er Failure of Nonsteroidal Aromatase Inhibitors: A Phase I ... Related Papers. J. Clin. Oncol. , 18, 2234, 10.1002/ppul.26417. - DOI - PubMed
LinkOut - more resources
Full Text Sources

