Potential for Aedes aegypti Larval Control and Environmental Friendliness of the Compounds Containing 2-Methyl-3,4-dihydroquinazolin-4-one Heterocycle
- PMID: 37483229
- PMCID: PMC10357533
- DOI: 10.1021/acsomega.3c01686
Potential for Aedes aegypti Larval Control and Environmental Friendliness of the Compounds Containing 2-Methyl-3,4-dihydroquinazolin-4-one Heterocycle
Abstract
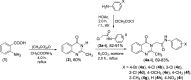
2-Methylquinazolin-4(3H)-one was prepared by the reaction of anthranilic acid, acetic anhydride, and ammonium acetate. The reaction of 2-methylquinazolin-4(3H)-one with N-aryl-2-chloroacetamides in acetone in the presence of potassium carbonate gave nine N-aryl-2-(2-methyl-4-oxoquinazolin-3(4H)-yl)acetamide compounds. The structures of these compounds were elucidated on the basis of their IR, 1H nuclear magnetic resonance (NMR), 13C NMR, and high-resolution mass spectrometry (HR-MS) spectral data. These synthesized compounds containing the 2-methyl-3,4-dihydroquinazolin-4-one moiety exhibited activity against Aedes aegypti mosquito larvae with LC50 values of 2.085-4.201 μg/mL after 72 h exposure, which is also confirmed using a quantitative structure-activity relationship (QSAR) model. Interestingly, these compounds did not exhibit toxicity to the nontarget organism Diplonychus rusticus. In silico molecular docking revealed acetylcholine binding protein (AChBP) and acetylcholinesterase (AChE) to be potential molecular targets. These data indicated the larvicidal potential and environmental friendliness of these N-aryl-2-(2-methyl-4-oxoquinazolin-3(4H)-yl)acetamide derivatives.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
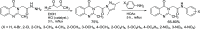




References
-
- El-Badry Y. A.; Anter N. A.; El-Sheshtawy H. S. Synthesis and evaluation of new polysubstituted quinazoline derivatives as potential antimicrobial agents. Der. Pharma Chem. 2012, 4, 1361–1370.
-
- Abd El-Samii Z. K.; El Feky S. A.; Abd El Fattah H. A. Design, synthesis and molecular modeling study of quinazolin-4 (3H) ones with potential anti-inflammatory activity. Biosci. Biotechnol. Res. Asia 2016, 6, 63–74.
-
- Helali A. Y. H.; Sarg M. T. M.; Koraa M. M. S.; El-Zoghbi M. S. F. Utility of 2-methyl-quinazolin-4 (3H)-one in the synthesis of heterocyclic compounds with anticancer activity. Open J. Med. Chem. 2014, 04, 12–37. 10.4236/ojmc.2014.41002. - DOI
LinkOut - more resources
Full Text Sources

