Loss of soluble guanylyl cyclase in platelets contributes to atherosclerotic plaque formation and vascular inflammation
- PMID: 37484062
- PMCID: PMC10361702
- DOI: 10.1038/s44161-022-00175-w
Loss of soluble guanylyl cyclase in platelets contributes to atherosclerotic plaque formation and vascular inflammation
Abstract
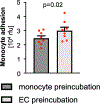
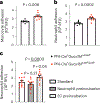
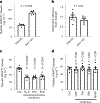
Variants in genes encoding the soluble guanylyl cyclase (sGC) in platelets are associated with coronary artery disease (CAD) risk. Here, by using histology, flow cytometry and intravital microscopy, we show that functional loss of sGC in platelets of atherosclerosis-prone Ldlr-/- mice contributes to atherosclerotic plaque formation, particularly via increasing in vivo leukocyte adhesion to atherosclerotic lesions. In vitro experiments revealed that supernatant from activated platelets lacking sGC promotes leukocyte adhesion to endothelial cells (ECs) by activating ECs. Profiling of platelet-released cytokines indicated that reduced platelet angiopoietin-1 release by sGC-depleted platelets, which was validated in isolated human platelets from carriers of GUCY1A1 risk alleles, enhances leukocyte adhesion to ECs. I mp or ta ntly, p ha rm ac ol ogical sGC stimulation increased platelet angiopoietin-1 release in vitro and reduced leukocyte recruitment and atherosclerotic plaque formation in atherosclerosis-prone Ldlr-/- mice. Therefore, pharmacological sGC stimulation might represent a potential therapeutic strategy to prevent and treat CAD.
Conflict of interest statement
Competing interests H.S. has received personal fees from MSD Sharp & Dohme, Amgen, Bayer Vital GmbH, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Servier, Brahms, Bristol Myers Squibb, Medtronic, Sanofi Aventis, Synlab, Pfizer and Vifor T as well as grants and personal fees from AstraZeneca that are unrelated to the submitted work. H.S. and T.K. are named inventors on a patent application for the prevention of restenosis after angioplasty and stent implantation (patent applicants: Klinikum rechts der Isar, German Heart Centre Munich; inventors: T. Kessler, A. Kastrati, H. Schunkert; application no. PCT/EP2021/053116; status: pending), which is unrelated to the submitted work. T.K. received lecture fees from Bayer HealthCare Pharmaceuticals. L.D., F.W. and P.S. are full-time employees of Bayer HealthCare Pharmaceuticals. The other authors declare no competing interests.
Figures




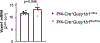








References
-
- Benjamin EJ et al. Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation 139, e56–e528 (2019). - PubMed
-
- Erdmann J, Kessler T, Venegas LM & Schunkert H A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc. Res. 114, 1241–1257 (2018). - PubMed
-
- Dang TA, Schunkert H & Kessler T cGMP signaling in cardiovascular diseases: linking genotype and phenotype. J. Cardiovasc. Pharmacol. 75, 516–525 (2020). - PubMed
-
- Erdmann J et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 504, 432–436 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
