Physiological and molecular responses of different rose (Rosa hybrida L.) cultivars to elevated ozone levels
- PMID: 37484545
- PMCID: PMC10359767
- DOI: 10.1002/pld3.513
Physiological and molecular responses of different rose (Rosa hybrida L.) cultivars to elevated ozone levels
Abstract
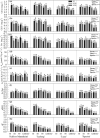
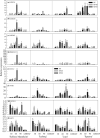
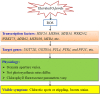
The increasing ground-level ozone (O3) pollution resulting from rapid global urbanization and industrialization has negative effects on many plants. Nonetheless, many gaps remain in our knowledge of how ornamental plants respond to O3. Rose (Rosa hybrida L.) is a commercially important ornamental plant worldwide. In this study, we exposed four rose cultivars ("Schloss Mannheim," "Iceberg," "Lüye," and "Spectra") to either unfiltered ambient air (NF), unfiltered ambient air plus 40 ppb O3 (NF40), or unfiltered ambient air plus 80 ppb O3 (NF80). Only the cultivar "Schloss Mannheim" showed significant O3-related effects, including foliar injury, reduced chlorophyll content, reduced net photosynthetic rate, reduced stomatal conductance, and reduced stomatal apertures. In "Schloss Mannheim," several transcription factor genes-HSF, WRKY, and MYB genes-were upregulated by O3 exposure, and their expression was correlated with that of NCED1, PP2Cs, PYR/PYL, and UGTs, which are related to ABA biosynthesis and signaling. These results suggest that HSF, WRKY, and MYB transcription factors and ABA are important components of the plant response to O3 stress, suggesting a possible strategy for cultivating O3-tolerant rose varieties.
Keywords: molecular response; ozone; physiological responses; rose; sensitivity.
© 2023 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Ashrafuzzaman, M. , Haque, Z. , Ali, B. , Mathew, B. , Yu, P. , Hochholdinger, F. , de Abreu Neto, J. B. , McGillen, M. R. , Ensikat, H. J. , & Manning, W. J. (2018). Ethylenediurea (EDU) mitigates the negative effects of ozone in rice: Insights into its mode of action. Plant, Cell & Environment, 41, 2882–2898. 10.1111/pce.13423 - DOI - PubMed
-
- Beijing Gardening and Greening Bureau . (2017). Collection of Beijing urban gardening and afforestation survey, monograph. Beijing Publishing House.
-
- Carvalho, D. R. A. , Vasconcelos, M. W. , Lee, S. , Koning‐Boucoiran, C. F. S. , Vreugdenhil, D. , Krens, F. A. , Heuvelink, E. , & Carvalho, S. M. P. (2016). Gene expression and physiological responses associated to stomatal functioning in Rosa × hybrida grown at high relative air humidity. Plant Science, 253, 154–163. 10.1016/j.plantsci.2016.09.018 - DOI - PubMed
-
- Chappelka, A. H. , & Samuelson, L. J. (1998). Ambient ozone effects on forest trees of the eastern United States: A review. New Phytologist, 139, 91–108. 10.1046/j.1469-8137.1998.00166.x - DOI
LinkOut - more resources
Full Text Sources

