Metabolite-sensing GPCRs controlling interactions between adipose tissue and inflammation
- PMID: 37484963
- PMCID: PMC10357040
- DOI: 10.3389/fendo.2023.1197102
Metabolite-sensing GPCRs controlling interactions between adipose tissue and inflammation
Abstract
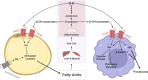
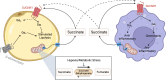
Metabolic disorders including obesity, diabetes and non-alcoholic steatohepatitis are a group of conditions characterised by chronic low-grade inflammation of metabolic tissues. There is now a growing appreciation that various metabolites released from adipose tissue serve as key signalling mediators, influencing this interaction with inflammation. G protein-coupled receptors (GPCRs) are the largest family of signal transduction proteins and most historically successful drug targets. The signalling pathways for several key adipose metabolites are mediated through GPCRs expressed both on the adipocytes themselves and on infiltrating macrophages. These include three main groups of GPCRs: the FFA4 receptor, which is activated by long chain free fatty acids; the HCA2 and HCA3 receptors, activated by hydroxy carboxylic acids; and the succinate receptor. Understanding the roles these metabolites and their receptors play in metabolic-immune interactions is critical to establishing how these GPCRs may be exploited for the treatment of metabolic disorders.
Keywords: G protein-coupled receptor; adipose; free fatty acid; hydroxy carboxylic acids; inflammation; metabolite signalling; succinate.
Copyright © 2023 Duncan, Vita, Dibnah and Hudson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- International Diabetes Federation . IDF diabetes atlas 2021, in: IDF diabetes atlas (2021). Available at: https://diabetesatlas.org/atlas/tenth-edition/ (Accessed 23, 2023).
-
- Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, et al. . Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab (2007) 92:2240–7. doi: 10.1210/JC.2006-1811 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

