Dual-ratio approach for detection of point fluorophores in biological tissue
- PMID: 37484977
- PMCID: PMC10362801
- DOI: 10.1117/1.JBO.28.7.077001
Dual-ratio approach for detection of point fluorophores in biological tissue
Abstract
Significance: Diffuse in vivo flow cytometry (DiFC) is an emerging fluorescence sensing method to non-invasively detect labeled circulating cells in vivo. However, due to signal-to-noise ratio (SNR) constraints largely attributed to background tissue autofluorescence (AF), DiFC's measurement depth is limited.
Aim: The dual ratio (DR)/dual slope is an optical measurement method that aims to suppress noise and enhance SNR to deep tissue regions. We aim to investigate the combination of DR and near-infrared (NIR) DiFC to improve circulating cells' maximum detectable depth and SNR.
Approach: Phantom experiments were used to estimate the key parameters in a diffuse fluorescence excitation and emission model. This model and parameters were implemented in Monte Carlo to simulate DR DiFC while varying noise and AF parameters to identify the advantages and limitations of the proposed technique.
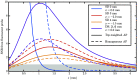
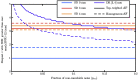
Results: Two key factors must be true to give DR DiFC an advantage over traditional DiFC: first, the fraction of noise that DR methods cannot cancel cannot be above the order of 10% for acceptable SNR. Second, DR DiFC has an advantage, in terms of SNR, if the distribution of tissue AF contributors is surface-weighted.
Conclusions: DR cancelable noise may be designed (e.g., through the use of source multiplexing), and indications point to the AF contributors' distribution being truly surface-weighted in vivo. Successful and worthwhile implementation of DR DiFC depends on these considerations, but results point to DR DiFC having possible advantages over traditional DiFC.
Keywords: Monte-Carlo methods; autofluorescence; dual ratio/dual slope; flow-cytometry; fluorescence; signal-to-noise ratio.
© 2023 The Authors.
Figures




Update of
-
Dual-ratio approach for detection of point fluorophores in biological tissue.ArXiv [Preprint]. 2023 Jul 3:arXiv:2305.14436v2. ArXiv. 2023. Update in: J Biomed Opt. 2023 Jul;28(7):077001. doi: 10.1117/1.JBO.28.7.077001. PMID: 37292468 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

