Revisiting the basis of the excited states for solvated fluorinated benzenes and pentafluorophenylalanine
- PMID: 37485304
- PMCID: PMC10361408
- DOI: 10.1016/j.rechem.2023.100770
Revisiting the basis of the excited states for solvated fluorinated benzenes and pentafluorophenylalanine
Abstract
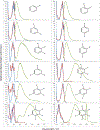
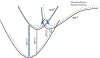
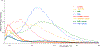
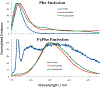
Vapor-phase molecular simulation studies of aromatic compounds with five or more fluorine atoms on the ring reveal emission spectra characterized by S0 → πσ* and πσ*→S0 transitions. In this study, the absorption, excitation, and solvent-dependent emission spectra of fluorinated benzenes, including pentaflurophenyalanine (F5Phe), which is a potential marker for biochemical research, were collected and compared to the results of the simulation. Time-dependent self-consistent field (TD-SCF) density functional theory (DFT) calculations were performed to examine the nature of excited states and relevant photo-physical processes. The results show that pentafluorobenzene (PFB) and hexafluorobenzene (HFB) show behavior consistent with the vapor phase simulation studies, that tracts well with benzenes substituted with fewer fluorine atoms. For example, 1,2,3-trifluorobenzene (123TFB) and 1,2,3,4-tetrafluorobenzene (1234TFB) show emission spectra with varying intensities of tails and shoulders. Those features are attributed to πσ*→S0 transitions where the πσ* state has been stabilized in the presence of solvents like water, acetonitrile, and isopropanol, which are different from their simulated behavior in the gas phase. The emission in water solvent especially shows a significant increase in the emission intensity at 310 nm, which is common for all studied samples. The emission spectrum of F5Phe closely reflects that of PFB, which arises from the interplay of both ππ *→S0 and πσ*→S0 transitions. Also, it is observed that the interaction between adjacent σ* orbitals of C-F bond for 123-TFB, 1234-TFB, 12345-PFB, and 123456-HFB contributes to further narrowing the energy gap between S0 and S1 states with a significant red shift on the emission spectra compared to their isomers.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


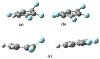


References
-
- Dichiarante V, Milani R, Metrangolo P, Natural surfactants towards a more sustainable fluorine chemistry, Green Chem 20 (2018) 13–27.
-
- Gillis E. p.; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Application of fluorine in medicinal chemistry. J. Med. Chem 2015, 58, 8315–8359. - PubMed
-
- Wang BC, Wang LJ, Jiang B, Wang SY, Wu N, Li XQ, Shi DY, Application of fluorine in drug design during 2010–2015 years: A mini-review, Mini Rev. Med. Chem 17 (2017) 683–692. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
