Preclinical evaluations of Pfs25-EPA and Pfs230D1-EPA in AS01 for a vaccine to reduce malaria transmission
- PMID: 37485364
- PMCID: PMC10359932
- DOI: 10.1016/j.isci.2023.107192
Preclinical evaluations of Pfs25-EPA and Pfs230D1-EPA in AS01 for a vaccine to reduce malaria transmission
Abstract
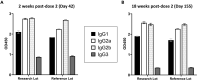
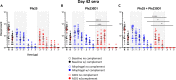
Malaria transmission-blocking vaccine candidates Pfs25-EPA and Pfs230D1-EPA target sexual stage development of Plasmodium falciparum parasites in the mosquito host, thereby reducing mosquito infectivity. When formulated on Alhydrogel, Pfs25-EPA has demonstrated safety and immunogenicity in a phase 1 field trial, while Pfs230D1-EPA has shown superior activity to Pfs25-EPA in a phase 1 US trial and has entered phase 2 field trials. Development continues to enhance immunogenicity of these candidates toward producing a vaccine to reduce malaria transmission (VRMT) with both pre-erythrocytic (i.e., anti-infection) and transmission-blocking components. GSK Adjuvant Systems have demonstrated successful potency in pre-erythrocytic vaccine trials and might offer a common platform for VRMT development. Here, we describe preclinical evaluations of Pfs25-EPA and Pfs230D1-EPA nanoparticles with GSK platforms. Formulations were stable after a series of assessments and induced superior antibody titers and functional activity in CD-1 mice, compared to Alhydrogel formulations of the same antigens.
Keywords: Molecular biology; Parasitology.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- WHO . World Health Organization; 2021. World Malaria Report 2021.https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
-
- Wu Y., Ellis R.D., Shaffer D., Fontes E., Malkin E.M., Mahanty S., Fay M.P., Narum D., Rausch K., Miles A.P., et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002636. - DOI - PMC - PubMed
-
- Sidén-Kiamos I., Vlachou D., Margos G., Beetsma A., Waters A.P., Sinden R.E., Louis C. Distinct roles for pbs21 and pbs25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J. Cell Sci. 2000;113:3419–3426. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

