Influence of conservation agriculture-based production systems on bacterial diversity and soil quality in rice-wheat-greengram cropping system in eastern Indo-Gangetic Plains of India
- PMID: 37485518
- PMCID: PMC10356824
- DOI: 10.3389/fmicb.2023.1181317
Influence of conservation agriculture-based production systems on bacterial diversity and soil quality in rice-wheat-greengram cropping system in eastern Indo-Gangetic Plains of India
Abstract
Introduction: Conservation agriculture (CA) is gaining attention in the South Asia as an environmentally benign and sustainable food production system. The knowledge of the soil bacterial community composition along with other soil properties is essential for evaluating the CA-based management practices for achieving the soil environment sustainability and climate resilience in the rice-wheat-greengram system. The long-term effects of CA-based tillage-cum-crop establishment (TCE) methods on earthworm population, soil parameters as well as microbial diversity have not been well studied.
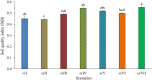
Methods: Seven treatments (or scenarios) were laid down with the various tillage (wet, dry, or zero-tillage), establishment method (direct-or drill-seeding or transplantation) and residue management practices (mixed with the soil or kept on the soil surface). The soil samples were collected after 7 years of experimentation and analyzed for the soil quality and bacterial diversity to examine the effect of tillage-cum-crop establishment methods.
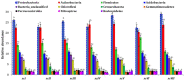
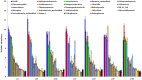
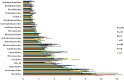
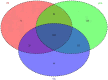
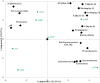
Results and discussion: Earthworm population (3.6 times), soil organic carbon (11.94%), macro (NPK) (14.50-23.57%) and micronutrients (Mn, and Cu) (13.25 and 29.57%) contents were appreciably higher under CA-based TCE methods than tillage-intensive farming practices. Significantly higher number of OTUs (1,192 ± 50) and Chao1 (1415.65 ± 14.34) values were observed in partial CA-based production system (p ≤ 0.05). Forty-two (42) bacterial phyla were identified across the scenarios, and Proteobacteria, Actinobacteria, and Firmicutes were the most dominant in all the scenarios. The CA-based scenarios harbor a high abundance of Proteobacteria (2-13%), whereas the conventional tillage-based scenarios were dominated by the bacterial phyla Acidobacteria and Chloroflexi and found statistically differed among the scenarios (p ≤ 0.05). Composition of the major phyla, i.e., Proteobacteria, Actinobacteria, and Firmicutes were associated differently with either CA or farmers-based tillage management practices. Overall, the present study indicates the importance of CA-based tillage-cum-crop establishment methods in shaping the bacterial diversity, earthworms population, soil organic carbon, and plant nutrient availability, which are crucial for sustainable agricultural production and resilience in agro-ecosystem.
Keywords: DNA sequencing; bacterial diversity; conservation agriculture; earthworm; metagenomics; rice-wheat-greengram; soil quality.
Copyright © 2023 Kumar, Choudhary, Naik, Mondal, Mishra, Poonia, Kumar, Hans, Kumar, Das, Kumar, Bhatt, Chaudhari, Malik, Craufurd, McDonald and Sherpa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahmad N., Johri S., Abdin M. Z., Qazi G. N. (2009). Molecular characterization of bacterial population in the forest soil of Kashmir, India. World J. Microbiol. Biotechnol. 25, 107–113. doi: 10.1007/s11274-008-9868-2 - DOI
-
- Aislabie J., Bockheim J., Mcleod M., Hunter D., Stevenson B., Barker G. M. (2012). Microbial biomass and community structure changes along a soil development chronosequence near Lake Wellman, southern Victoria land. Antarct. Sci. 24, 154–164. doi: 10.1017/S0954102011000873 - DOI
-
- Andrews S. S., Karlen D. L., Mitchell J. P. (2002). A comparison of soil quality indexing methods for vegetable production systems in northern California. Agric. Ecosyst. Environ. 90, 25–45. doi: 10.1016/S0167-8809(01)00174-8 - DOI
-
- Ashworth A. J., Allen F. L., Tyler D. D., Pote D. H., Shipitalo M. J. (2017). Earthworm populations are affected from long-term crop sequences and bio-covers under no-tillage. Pedobiologia 60, 27–33. doi: 10.1016/j.pedobi.2017.01.001 - DOI
-
- Babin D., Deubel A., Jacquiod S., Sørensen S. J., Geistlinger J., Grosch R., et al. (2019). Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol. Biochem. 129, 17–28. doi: 10.1016/j.soilbio.2018.11.002 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

