CD9 co-operation with syndecan-1 is required for a major staphylococcal adhesion pathway
- PMID: 37486132
- PMCID: PMC10470606
- DOI: 10.1128/mbio.01482-23
CD9 co-operation with syndecan-1 is required for a major staphylococcal adhesion pathway
Abstract
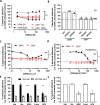
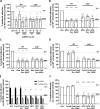
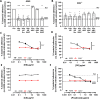
Epithelial colonization is a critical first step in bacterial pathogenesis. Staphylococcus aureus can utilize several host factors to associate with cells, including α5β1 integrin and heparan sulfate proteoglycans, such as the syndecans. Here, we demonstrate that a partner protein of both integrins and syndecans, the host membrane adapter protein tetraspanin CD9, is essential for syndecan-mediated staphylococcal adhesion. Fibronectin is also essential in this process, while integrins are only critical for post-adhesion entry into human epithelial cells. Treatment of epithelial cells with CD9-derived peptide or heparin caused significant reductions in staphylococcal adherence, dependent on both CD9 and syndecan-1. Exogenous fibronectin caused a CD9-dependent increase in staphylococcal adhesion, whereas blockade of β1 integrins did not affect adhesion but did reduce the subsequent internalization of adhered bacteria. CD9 disruption or deletion increased β1 integrin-mediated internalization, suggesting that CD9 coordinates sequential staphylococcal adhesion and internalization. CD9 controls staphylococcal adhesion through syndecan-1, using a mechanism that likely requires CD9-mediated syndecan organization to correctly display fibronectin at the host cell surface. We propose that CD9-derived peptides or heparin analogs could be developed as anti-adhesion treatments to inhibit the initial stages of staphylococcal pathogenesis. IMPORTANCE Staphylococcus aureus infection is a significant cause of disease and morbidity. Staphylococci utilize multiple adhesion pathways to associate with epithelial cells, including interactions with proteoglycans or β1 integrins through a fibronectin bridge. Interference with another host protein, tetraspanin CD9, halves staphylococcal adherence to epithelial cells, although CD9 does not interact directly with bacteria. Here, we define the role of CD9 in staphylococcal adherence and uptake, observing that CD9 coordinates syndecan-1, fibronectin, and β1 integrins to allow efficient staphylococcal infection. Two treatments that disrupt this action are effective and may provide an alternative to antibiotics. We provide insights into the mechanisms that underlie staphylococcal infection of host cells, linking two known adhesion pathways together through CD9 for the first time.
Keywords: CD9; HSPG; Staphylococcus aureus; bacterial adhesion; epithelial; fibronectin; syndecan; tetraspanin.
Conflict of interest statement
P.N.M., R.I., and B.C. are co-inventors on patent WO2021175809A1, related to the peptides used in this study. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
