Impact of Latanoprost Antiglaucoma Eyedrops and Their Excipients on Toxicity and Healing Characteristics in the Ex Vivo Eye Irritation Test System
- PMID: 37486574
- PMCID: PMC10441994
- DOI: 10.1007/s40123-023-00769-y
Impact of Latanoprost Antiglaucoma Eyedrops and Their Excipients on Toxicity and Healing Characteristics in the Ex Vivo Eye Irritation Test System
Abstract
Introduction: Corneal epithelial toxicity and delayed healing process have already been attributed to preservatives or some excipients. We study the effects of galenic components in antiglaucoma drugs such as benzalkonium chloride (BAC) or surfactants like macrogolglycerol hydroxystearate 40 (MGHS 40) on corneal toxicity in an ex vivo system mimicking chronic use.
Methods: Latanoprost-containing eyedrops are available with and without preservatives on the market. Unpreserved, they are available in different formulations with various excipients like MGHS at different concentrations (0%, 2.5%, and 5%). We studied these in the ex vivo bioreactor (EVEIT) on initially injured rabbit corneas. The drugs were applied six times daily for observation periods of 3 or 5 days. BAC, 5% MGHS 40 solution, and 0.18% hyaluronic acid served as controls. Macroscopic photographic, biochemical methods and corneal integrity quantification were used for evaluation. Toxicity was assessed by measuring wound healing and corneal fluorescein sodium permeability and was confirmed by histology studies.
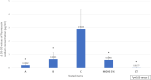
Results: The BAC-preserved formulation resulted in high corneal toxicity, which was expected. Interestingly, the preservative-free (PF) formulation containing 5% MGHS 40, carbomer, macrogol 4000, and sorbitol showed the highest corneal toxicity, followed by the control formulation with equal MGHS 40 concentration, which presented significantly less damage. No toxicity was shown by eyedrops containing 2.5% MGHS 40 or salts only.
Conclusion: Our study demonstrates a significant corneal toxicity of certain formulations of PF antiglaucoma ophthalmic drugs containing 5% MGHS 40 with other excipients compared to other formulations with lower MGHS 40 concentrations (2.5% or 0%), or even compared to the solution containing 5% MGHS alone. This suggests a concentration-dependent toxicity of MGHS 40, especially in interaction with other excipients, which may increase its epithelial toxicity, and that has to be considered in clinical glaucoma therapy. Further single-component formulation trials are needed to support this interpretation.
Keywords: BAC; Corneal healing; Cytotoxicity; Excipient; Ex vivo model; Glaucoma medication; Latanoprost; Macrogolglycerol hydroxystearate 40; Preservative.
© 2023. The Author(s).
Conflict of interest statement
Claudia Panfil and Norbert Schrage have nothing to disclose. Laure Chauchat, Camille Guerin, Hayetta Rebika and Marwan Sahyoun declare working for Horus Pharma at the time of completion of the manuscript.
Figures







References
-
- European Glaucoma Society. European Glaucoma Society terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021;105:1–169. - PubMed
LinkOut - more resources
Full Text Sources

