Redefining the human corneal immune compartment using dynamic intravital imaging
- PMID: 37487076
- PMCID: PMC10400993
- DOI: 10.1073/pnas.2217795120
Redefining the human corneal immune compartment using dynamic intravital imaging
Abstract
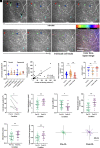
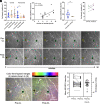
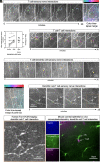
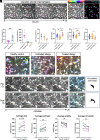
The healthy human cornea is a uniquely transparent sensory tissue where immune responses are tightly controlled to preserve vision. The cornea contains immune cells that are widely presumed to be intraepithelial dendritic cells (DCs). Corneal immune cells have diverse cellular morphologies and morphological alterations are used as a marker of inflammation and injury. Based on our imaging of corneal T cells in mice, we hypothesized that many human corneal immune cells commonly defined as DCs are intraepithelial lymphocytes (IELs). To investigate this, we developed functional in vivo confocal microscopy (Fun-IVCM) to investigate cell dynamics in the human corneal epithelium and stroma. We show that many immune cells resident in the healthy human cornea are T cells. These corneal IELs are characterized by rapid, persistent motility and interact with corneal DCs and sensory nerves. Imaging deeper into the corneal stroma, we show that crawling macrophages and rare motile T cells patrol the tissue. Furthermore, we identify altered immune cell behaviors in response to short-term contact lens wear (acute inflammatory stimulus), as well as in individuals with allergy (chronic inflammatory stimulus) that was modulated by therapeutic intervention. These findings redefine current understanding of immune cell subsets in the human cornea and reveal how resident corneal immune cells respond and adapt to chronic and acute stimuli.
Keywords: T cell; confocal; cornea; eye; macrophage.
Conflict of interest statement
L.E.D., X.Z., S.N.M., and H.R.C. are inventors on a patent relating to the imaging method described in this paper. National Health and Medical Research Council (NHMRC), APP1126540, funded the mouse experiments in this paper. There was no specific funding for the other aspects of this work.
Figures


References
-
- Chinnery H. R., Zhang X. Y., Wu C. Y., Downie L. E., Corneal immune cell morphometry as an indicator of local and systemic pathology: A review. Clin. Exp. Ophthalmol. 49, 729–740 (2021). - PubMed
-
- Hamrah P., Zhang Q., Liu Y., Dana M. R., Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest. Ophthalmol. Vis. Sci. 43, 639–646 (2002). - PubMed
-
- Hamrah P., Huq S. O., Liu Y., Zhang Q., Dana M. R., Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 74, 172–178 (2003). - PubMed
-
- Hamrah P., Liu Y., Zhang Q., Dana M. R., The corneal stroma is endowed with a significant number of resident dendritic cells. Invest. Ophthalmol. Vis. Sci. 44, 581–589 (2003). - PubMed
-
- Jiao H., et al. , Topographical and morphological differences of corneal dendritic cells during steady state and inflammation. Ocul. Immunol. Inflamm. 28, 898–907 (2020). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

