Molecular Classification Predicts Response to Radiotherapy in the Randomized PORTEC-1 and PORTEC-2 Trials for Early-Stage Endometrioid Endometrial Cancer
- PMID: 37487144
- PMCID: PMC10522107
- DOI: 10.1200/JCO.23.00062
Molecular Classification Predicts Response to Radiotherapy in the Randomized PORTEC-1 and PORTEC-2 Trials for Early-Stage Endometrioid Endometrial Cancer
Abstract
Purpose: The molecular classification of endometrial cancer (EC) has proven to have prognostic value and is predictive of response to adjuvant chemotherapy. Here, we investigate its predictive value for response to external beam radiotherapy (EBRT) and vaginal brachytherapy (VBT) in early-stage endometrioid EC (EEC).
Methods: Data of the randomized PORTEC-1 trial (n = 714) comparing pelvic EBRT with no adjuvant therapy in early-stage intermediate-risk EC and the PORTEC-2 trial (n = 427) comparing VBT with EBRT in early-stage high-intermediate-risk EC were used. Locoregional (including vaginal and pelvic) recurrence-free survival was compared between treatment groups across the four molecular classes using Kaplan-Meier's methodology and log-rank tests.
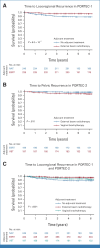
Results: A total of 880 molecularly classified ECs, 484 from PORTEC-1 and 396 from PORTEC-2, were included. The majority were FIGO-2009 stage I EEC (97.2%). The median follow-up was 11.3 years. No locoregional recurrences were observed in EC with a pathogenic mutation of DNA polymerase-ε (POLEmut EC). In mismatch repair-deficient (MMRd) EC, locoregional recurrence-free survival was similar after EBRT (94.2%), VBT (94.2%), and no adjuvant therapy (90.3%; P = .74). In EC with a p53 abnormality (p53abn EC), EBRT (96.9%) had a substantial benefit over VBT (64.3%) and no adjuvant therapy (72.2%; P = .048). In EC with no specific molecular profile (NSMP EC), both EBRT (98.3%) and VBT (96.2%) yielded better locoregional control than no adjuvant therapy (87.7%; P < .0001).
Conclusion: The molecular classification of EC predicts response to radiotherapy in stage I EEC and may guide adjuvant treatment decisions. Omitting radiotherapy seems to be safe in POLEmut EC. The benefit of radiotherapy seems to be limited in MMRd EC. EBRT yields a significantly better locoregional recurrence-free survival than VBT or no adjuvant therapy in p53abn EC. VBT is the treatment of choice for NSMP EC as it is as effective as EBRT and significantly better than no adjuvant therapy for locoregional tumor control.
Trial registration: ClinicalTrials.gov NCT00376844.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- WHO Classification of Tumours Editorial Board : WHO classification of tumours (ed 5), in Female Genital Tumours, Lyon, France, International Agency for Research on Cancer. Volume 4, 2020, pp 252-266
-
- Stelloo E, Nout RA, Osse EM, et al. : Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 22:4215-4224, 2016 - PubMed
-
- Kommoss S, McConechy MK, Kommoss F, et al. : Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29:1180-1188, 2018 - PubMed
-
- Talhouk A, McConechy MK, Leung S, et al. : Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 123:802-813, 2017 - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

