Anandamide modulates WNT-5A/BCL-2, IP3/NFATc1, and HMGB1/NF-κB trajectories to protect against mercuric chloride-induced acute kidney injury
- PMID: 37488162
- PMCID: PMC10366223
- DOI: 10.1038/s41598-023-38659-9
Anandamide modulates WNT-5A/BCL-2, IP3/NFATc1, and HMGB1/NF-κB trajectories to protect against mercuric chloride-induced acute kidney injury
Abstract
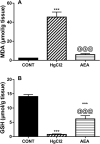
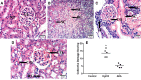
Endocannabinoid anandamide (AEA) has a physiological role in regulating renal blood flow, whereas its analogs ameliorated renal ischemia/reperfusion injury. Nonetheless, the role of AEA against mercuric chloride (HgCl2)-induced renal toxicity has not been unraveled. Rats were allocated into control, HgCl2, and HgCl2/AEA treated groups. The administration of AEA quelled the HgCl2-mediated increase in inositol trisphosphate (IP3) and nuclear factor of activated T-cells cytoplasmic 1 (NFATc1). The endocannabinoid also signified its anti-inflammatory potential by turning off the inflammatory cascade evidenced by the suppression of high mobility group box protein-1 (HMGB1), receptor of glycated end products (RAGE), nuclear factor-κB p65 (NF-κB), and unexpectedly PPAR-γ. Additionally, the aptitude of AEA to inhibit malondialdehyde and boost glutathione points to its antioxidant capacity. Moreover, AEA by enhancing the depleted renal WNT-5A and reducing cystatin-C and KIM-1 (two kidney function parameters) partly verified its anti-apoptotic capacity, confirmed by inhibiting caspase-3 and increasing B-cell lymphoma-2 (BCL-2). The beneficial effect of AEA was mirrored by the improved architecture and kidney function evidenced by the reduction in cystatin-C, KIM-1, creatinine, BUN, and caspase1-induced activated IL-18. In conclusion, our results verify the reno-protective potential of AEA against HgCl2-induced kidney injury by its anti-inflammatory, antioxidant, and anti-apoptotic capacities by modulating WNT-5A/BCL-2, IP3/NFATC1, HMGB-1/RAGE/NF-κB, caspase-1/IL-18, and caspase-3/BCL-2 cues.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

