Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct
- PMID: 37488172
- PMCID: PMC10366142
- DOI: 10.1038/s41598-023-39102-9
Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct
Abstract
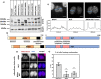
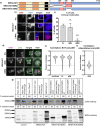
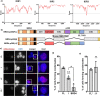
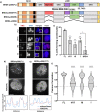
Aberrant formation of biomolecular condensates has been proposed to play a role in several cancers. The oncogenic fusion protein BRD4-NUT forms condensates and drives changes in gene expression in Nut Carcinoma. Here we sought to understand the molecular elements of BRD4-NUT and its associated histone acetyltransferase (HAT), p300, that promote these activities. We determined that a minimal fragment of NUT (MIN) in fusion with BRD4 is necessary and sufficient to bind p300 and form condensates. Furthermore, a BRD4-p300 fusion protein also forms condensates and drives gene expression similarly to BRD4-NUT(MIN), suggesting the p300 fusion may mimic certain features of BRD4-NUT. The intrinsically disordered regions, transcription factor-binding domains, and HAT activity of p300 all collectively contribute to condensate formation by BRD4-p300, suggesting that these elements might contribute to condensate formation by BRD4-NUT. Conversely, only the HAT activity of BRD4-p300 appears necessary to mimic the transcriptional profile of cells expressing BRD4-NUT. Our results suggest a model for condensate formation by the BRD4-NUT:p300 complex involving a combination of positive feedback and phase separation, and show that multiple overlapping, yet distinct, regions of p300 contribute to condensate formation and transcriptional regulation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct.bioRxiv [Preprint]. 2023 May 11:2023.05.11.540414. doi: 10.1101/2023.05.11.540414. bioRxiv. 2023. Update in: Sci Rep. 2023 Jul 24;13(1):11907. doi: 10.1038/s41598-023-39102-9. PMID: 37214845 Free PMC article. Updated. Preprint.
References
-
- Shirnekhi HK, Chandra B, Kriwacki RW. The role of phase-separated condensates in fusion oncoprotein-driven cancers. Ann. Rev. Cancer Biol. 2023;7:73–91. doi: 10.1146/annurev-cancerbio-061421-122050. - DOI
-
- Chandra B, et al. Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. 2022;12(4):1152–1169. doi: 10.1158/2159-8290.CD-21-0674. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

