Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study
- PMID: 37488291
- PMCID: PMC10427429
- DOI: 10.1038/s41591-023-02455-9
Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study
Abstract
Anorexia nervosa (AN) is a deadly illness with no proven treatments to reverse core symptoms and no medications approved by the US Food and Drug Administration. Novel treatments are urgently needed to improve clinical outcomes. In this open-label feasibility study, 10 adult female participants (mean body mass index 19.7 kg m-2; s.d. 3.7) who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for AN or pAN (partial remission) were recruited to a study conducted at an academic clinical research institute. Participants received a single 25-mg dose of synthetic psilocybin in conjunction with psychological support. The primary aim was to assess safety, tolerability and feasibility at post-treatment by incidences and occurrences of adverse events (AEs) and clinically significant changes in electrocardiogram (ECG), laboratory tests, vital signs and suicidality. No clinically significant changes were observed in ECG, vital signs or suicidality. Two participants developed asymptomatic hypoglycemia at post-treatment, which resolved within 24 h. No other clinically significant changes were observed in laboratory values. All AEs were mild and transient in nature. Participants' qualitative perceptions suggest that the treatment was acceptable for most participants. Results suggest that psilocybin therapy is safe, tolerable and acceptable for female AN, which is a promising finding given physiological dangers and problems with treatment engagement. ClinicalTrials.gov identifier NCT04661514 .
© 2023. The Author(s).
Conflict of interest statement
S.K.P. is a senior clinical consultant for Compass Pathways, the funder of the study, and receives compensation for this role. Additionally, she is a sub-investigator for an upcoming Compass-sponsored randomized trial evaluating psilocybin for anorexia nervosa and is a lead therapist for this trial and other Compass-sponsored trials. W.H.K. has served as a consultant and advisor for Compass Pathways, the funder of this study, and has received compensation for these roles. W.H.K. has also received financial support for this study and for an upcoming randomized controlled trial evaluating psilocybin for anorexia nervosa, which is sponsored by Compass Pathways. Additionally, he serves as site principal investigator for the upcoming referenced trial. S.S., T.G., K.Y., A.B., J.T. and D.F.M. report no competing interests.
Figures

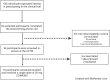
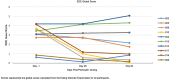
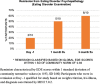
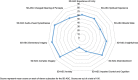
Comment in
-
Psilocybin for the treatment of anorexia nervosa.Nat Med. 2023 Aug;29(8):1906-1907. doi: 10.1038/s41591-023-02458-6. Nat Med. 2023. PMID: 37488290 No abstract available.
-
Psychedelic treatment for anorexia.Nat Rev Drug Discov. 2023 Sep;22(9):697. doi: 10.1038/d41573-023-00127-4. Nat Rev Drug Discov. 2023. PMID: 37532824 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

