An injectable, self-healing, electroconductive hydrogel loaded with neural stem cells and donepezil for enhancing local therapy effect of spinal cord injury
- PMID: 37488558
- PMCID: PMC10367392
- DOI: 10.1186/s13036-023-00368-2
An injectable, self-healing, electroconductive hydrogel loaded with neural stem cells and donepezil for enhancing local therapy effect of spinal cord injury
Abstract
Background: Spinal cord injury (SCI) is a serious injury with high mortality and disability rates, and there is no effective treatment at present. It has been reported that some treatments, such as drug intervention and stem cell transplantation have positive effects in promoting neurological recovery. Although those treatments are effective for nerve regeneration, many drawbacks, such as low stem cell survival rates and side effects caused by systemic medication, have limited their development. In recent years, injectable hydrogel materials have been widely used in tissue engineering due to their good biocompatibility, biodegradability, controllable properties, and low invasiveness. The treatment strategy of injectable hydrogels combined with stem cells or drugs has made some progress in SCI repair, showing the potential to overcome the drawbacks of traditional drugs and stem cell therapy.
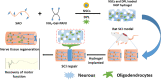
Methods: In this study, a novel injectable electroactive hydrogel (NGP) based on sodium hyaluronate oxide (SAO) and polyaniline-grafted gelatine (NH2-Gel-PANI) was developed as a material in which to load neural stem cells (NSCs) and donepezil (DPL) to facilitate nerve regeneration after SCI. To evaluate the potential of the prepared NGP hydrogel in SCI repair applications, the surface morphology, self-repairing properties, electrical conductivity and cytocompatibility of the resulting hydrogel were analysed. Meanwhile, we evaluated the neural repair ability of NGP hydrogels loaded with DPL and NSCs using a rat model of spinal cord injury.
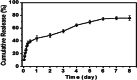
Results: The NGP hydrogel has a suitable pore size, good biocompatibility, excellent conductivity, and injectable and self-repairing properties, and its degradation rate matches the repair cycle of spinal cord injury. In addition, DPL could be released continuously and slowly from the NGP hydrogel; thus, the NGP hydrogel could serve as an excellent carrier for drugs and cells. The results of in vitro cell experiments showed that the NGP hydrogel had good cytocompatibility and could significantly promote the neuronal differentiation and axon growth of NSCs, and loading the hydrogel with DPL could significantly enhance this effect. More importantly, the NGP hydrogel loaded with DPL showed a significant inhibitory effect on astrocytic differentiation of NSCs in vitro. Animal experiments showed that the combination of NGP hydrogel, DPL, and NSCs had the best therapeutic effect on the recovery of motor function and nerve conduction function in rats. NGP hydrogel loaded with NSCs and DPL not only significantly increased the myelin sheath area, number of new neurons and axon area but also minimized the area of the cystic cavity and glial scar and promoted neural circuit reconstruction.
Conclusions: The DPL- and NSC-laden electroactive hydrogel developed in this study is an ideal biomaterial for the treatment of traumatic spinal cord injury.
Keywords: Conductivity; Donepezil; Hydrogel; Neural stem cells; Spinal cord injury.
© 2023. The Author(s).
Conflict of interest statement
There are no conflicts to declare.
Figures









References
-
- Ahuja CS et al. “Traumatic spinal cord injury,“ Nature Reviews Disease Primers, vol. 3, Apr 2017, Art no. 17018, doi: 10.1038/nrdp.2017.18. - PubMed
-
- Ahuja CS et al. “Traumatic Spinal Cord Injury-Repair and Regeneration,“ Neurosurgery, vol. 80, no. 3, pp. S9-S22, Mar 2017, doi: 10.1093/neuros/nyw080. - PubMed
-
- Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. Jun 2019;25(6):898–908. 10.1038/s41591-019-0475-6. - PubMed
-
- Alexanian AR, Fehlings MG, Zhang ZY, Maiman DJ. Transplanted Neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and locomotor recovery in spinal cord injured rats. Neurorehabilit Neural Repair. Nov-Dec 2011;25(9):873–80. 10.1177/1545968311416823. - PubMed
Grants and funding
- 20230508069RC/Jilin Province science and technology development Foundation of Jilin Provincial Science and Technology Department
- 20200201454JC/Natural Science Foundation of Jilin Provincial Science and Technology Department
- 20200201454JC/Natural Science Foundation of Jilin Provincial Science and Technology Department
- No.82171388/National Natural Science Foundation of China
- No.82171388/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

