The Restrictive Red Blood Cell Transfusion Strategy for Critically Injured Patients (RESTRIC) trial: a cluster-randomized, crossover, non-inferiority multicenter trial of restrictive transfusion in trauma
- PMID: 37488591
- PMCID: PMC10364403
- DOI: 10.1186/s40560-023-00682-3
The Restrictive Red Blood Cell Transfusion Strategy for Critically Injured Patients (RESTRIC) trial: a cluster-randomized, crossover, non-inferiority multicenter trial of restrictive transfusion in trauma
Abstract
Background: The efficacies of fresh frozen plasma and coagulation factor transfusion have been widely evaluated in trauma-induced coagulopathy management during the acute post-injury phase. However, the efficacy of red blood cell transfusion has not been adequately investigated in patients with severe trauma, and the optimal hemoglobin target level during the acute post-injury and resuscitation phases remains unclear. Therefore, this study aimed to examine whether a restrictive transfusion strategy was clinically non-inferior to a liberal transfusion strategy during the acute post-injury phase.
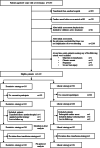
Methods: This cluster-randomized, crossover, non-inferiority multicenter trial was conducted at 22 tertiary emergency medical institutions in Japan and included adult patients with severe trauma at risk of major bleeding. The institutions were allocated a restrictive or liberal transfusion strategy (target hemoglobin levels: 7-9 or 10-12 g/dL, respectively). The strategies were applied to patients immediately after arrival at the emergency department. The primary outcome was 28-day survival after arrival at the emergency department. Secondary outcomes included transfusion volume, complication rates, and event-free days. The non-inferiority margin was set at 3%.
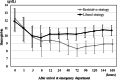
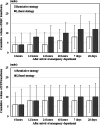
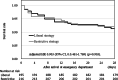
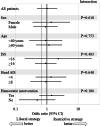
Results: The 28-day survival rates of patients in the restrictive (n = 216) and liberal (n = 195) strategy groups were 92.1% and 91.3%, respectively. The adjusted odds ratio for 28-day survival in the restrictive versus liberal strategy group was 1.02 (95% confidence interval: 0.49-2.13). Significant non-inferiority was not observed. Transfusion volumes and hemoglobin levels were lower in the restrictive strategy group than in the liberal strategy group. No between-group differences were noted in complication rates or event-free days.
Conclusions: Although non-inferiority of the restrictive versus liberal transfusion strategy for 28-day survival was not statistically significant, the mortality and complication rates were similar between the groups. The restrictive transfusion strategy results in a lower transfusion volume.
Trial registration number: umin.ac.jp/ctr: UMIN000034405, registration date: 8 October 2018.
Keywords: Hemoglobin; Red blood cell; Resuscitation; Transfusion; Trauma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. - DOI - PMC - PubMed
-
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/nejm199902113400601. - DOI - PubMed
LinkOut - more resources
Full Text Sources

