BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in vitro model of human fetal spinal cord development
- PMID: 37488910
- PMCID: PMC10503628
- DOI: 10.4103/1673-5374.373669
BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in vitro model of human fetal spinal cord development
Abstract
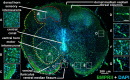
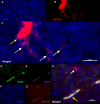
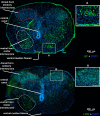
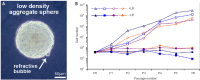
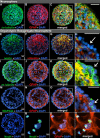
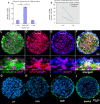
Roof plate secretion of bone morphogenetic proteins (BMPs) directs the cellular fate of sensory neurons during spinal cord development, including the formation of the ascending sensory columns, though their biology is not well understood. Type-II BMP receptor (BMPRII), the cognate receptor, is expressed by neural precursor cells during embryogenesis; however, an in vitro method of enriching BMPRII+ human neural precursor cells (hNPCs) from the fetal spinal cord is absent. Immunofluorescence was undertaken on intact second-trimester human fetal spinal cord using antibodies to BMPRII and leukemia inhibitory factor (LIF). Regions of highest BMPRII+ immunofluorescence localized to sensory columns. Parenchymal and meningeal-associated BMPRII+ vascular cells were identified in both intact fetal spinal cord and cortex by co-positivity with vascular lineage markers, CD34/CD39. LIF immunostaining identified a population of somas concentrated in dorsal and ventral horn interneurons, mirroring the expression of LIF receptor/CD118. A combination of LIF supplementation and high-density culture maintained culture growth beyond 10 passages, while synergistically increasing the proportion of neurospheres with a stratified, cytoarchitecture. These neurospheres were characterized by BMPRII+/MAP2ab+/-/βIII-tubulin+/nestin-/vimentin-/GFAP-/NeuN- surface hNPCs surrounding a heterogeneous core of βIII-tubulin+/nestin+/vimentin+/GFAP+/MAP2ab-/NeuN- multipotent precursors. Dissociated cultures from tripotential neurospheres contained neuronal (βIII-tubulin+), astrocytic (GFAP+), and oligodendrocytic (O4+) lineage cells. Fluorescence-activated cell sorting-sorted BMPRII+ hNPCs were MAP2ab+/-/βIII-tubulin+/GFAP-/O4- in culture. This is the first isolation of BMPRII+ hNPCs identified and characterized in human fetal spinal cords. Our data show that LIF combines synergistically with high-density reaggregate cultures to support the organotypic reorganization of neurospheres, characterized by surface BMPRII+ hNPCs. Our study has provided a new methodology for an in vitro model capable of amplifying human fetal spinal cord cell numbers for > 10 passages. Investigations of the role BMPRII plays in spinal cord development have primarily relied upon mouse and rat models, with interpolations to human development being derived through inference. Because of significant species differences between murine biology and human, including anatomical dissimilarities in central nervous system (CNS) structure, the findings made in murine models cannot be presumed to apply to human spinal cord development. For these reasons, our human in vitro model offers a novel tool to better understand neurodevelopmental pathways, including BMP signaling, as well as spinal cord injury research and testing drug therapies.
Keywords: BMPRII; bone morphogenetic protein; histotypic; human spinal cord development; leukemia inhibitory factor; neurosphere; organotypic; reaggregate; sensory columns.
Conflict of interest statement
None
Figures


References
-
- Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. (2002);105:1672–1678. - PubMed
-
- Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development:dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. (2002);61:197–211. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

