Tau, microtubule dynamics, and axonal transport: New paradigms for neurodegenerative disease
- PMID: 37489532
- PMCID: PMC10630968
- DOI: 10.1002/bies.202200138
Tau, microtubule dynamics, and axonal transport: New paradigms for neurodegenerative disease
Abstract
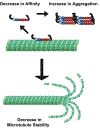
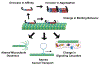
The etiology of Tauopathies, a diverse class of neurodegenerative diseases associated with the Microtubule Associated Protein (MAP) Tau, is usually described by a common mechanism in which Tau dysfunction results in the loss of axonal microtubule stability. Here, we reexamine and build upon the canonical disease model to encompass other Tau functions. In addition to regulating microtubule dynamics, Tau acts as a modulator of motor proteins, a signaling hub, and a scaffolding protein. This diverse array of functions is related to the dynamic nature of Tau isoform expression, post-translational modification (PTM), and conformational flexibility. Thus, there is no single mechanism that can describe Tau dysfunction. The effects of specific pathogenic mutations or aberrant PTMs need to be examined on all of the various functions of Tau in order to understand the unique etiology of each disease state.
Keywords: MAP-Tau; Tau; Tauopathy; microtubule; microtubule associated protein; neurodegeneration.
© 2023 The Authors. BioEssays published by Wiley Periodicals LLC.
Figures


References
-
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. (1994). Domains of tau protein and interactions with microtubules. Biochemistry, 33, 9511–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

