Tenecteplase real-world data: A three phase sequential comparison
- PMID: 37489615
- PMCID: PMC10683726
- DOI: 10.1177/23969873231187436
Tenecteplase real-world data: A three phase sequential comparison
Abstract
Introduction: The New Zealand (NZ) Central Region Stroke Network, serving 1.17 million catchment population, changed to tenecteplase for stroke thrombolysis in 2020 but was forced to revert to Alteplase in 2021 due to a sudden cessation of drug supply. We used this unique opportunity to assess for potential before and after temporal trend confounding.
Patients and methods: In NZ all reperfused patients are entered prospectively into a national database for safety monitoring. We assessed Central Region patient outcomes and treatment metrics over three time periods: alteplase use (January 2018-January 2020); during switch to tenecteplase (February 2020-February 2021) and after reverting to alteplase (February 2021-December 2022) adjusting regression analyses for hospital, age, onset-to-needle, NIHSS, pre-morbid mRS and thrombectomy.
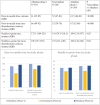
Results: Between January 2018 and December 2022, we treated 1121 patients with Alteplase and 286 with tenecteplase. Overall, patients treated with tenecteplase had greater odds of favorable outcome ordinal mRS [aOR = 1.43 (95% CI = 1.11-1.85)]; shorter door-to-needle (DTN) time [median 52 (IQR 47-83) vs 61 (45-84) minutes, p < 0.0001] and needle to groin (NTG) times [118 (74.5-218.5) vs 185 (118-255); p = 0.02)]. Symptomatic intracerebral hemorrhage (sICH) rate was lower in tenecteplase group [aOR 0.29 (0.09-0.95)]. Findings similarly favored tenecteplase when comparing tenecteplase to only the second alteplase phase. There was no inter-group difference when comparing the two alteplase phases.
Conclusions: Our results suggest that previously reported benefits from tenecteplase in a real-world setting were not likely attributable to a temporal confounding.
Keywords: Stroke; reperfusion; tenecteplase; thrombolysis.
© European Stroke Organisation 2023.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Katsanos AH, Safouris A, Sarraj A, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke 2021; 52: 308–312. - PubMed
-
- Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 2019; 50: 2156–2162. - PubMed
-
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linkes, randomised, controlled, non-inferiority trial. Lancet 2022; 400: 161–169. - PubMed
-
- Warach SJ, Ranta A, Kim J, et al. Symptomatic intracranial hemorrhage with tenecteplase versus alteplase in acute ischemic stroke – results from CERTAIN (The comparative effectiveness of routine tenecteplase versus alteplase in acute ischemic stoke). JAMA Neurol. Epub ahead of print 30 May 2023. DOI: 10.1001/jamaneurol.2023.1449 - DOI - PMC - PubMed
-
- Mahawish K, Gommans J, Kleinig T, et al. Switching to tenecteplase for stroke thrombolysis: Real-world experience and outcomes in a Regional Stroke Network. Stroke 2021; 52: e590–e593. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical