A Comparison of the Effects of Dexamethasone and Methylprednisolone, Used on Level-3 Intensive Care COVID-19 Patients, on Mortality: A Multi-Center Retrospective Study
- PMID: 37489719
- PMCID: PMC10366414
- DOI: 10.3346/jkms.2023.38.e232
A Comparison of the Effects of Dexamethasone and Methylprednisolone, Used on Level-3 Intensive Care COVID-19 Patients, on Mortality: A Multi-Center Retrospective Study
Abstract
Background: Coronavirus disease 2019 (COVID-19) is often a mild disease, usually manifesting with respiratory complaints, and is sometimes mortal due to multiple organ failure. Hyperinflammation is a known COVID-19 component and is associated with organ dysfunction, disease severity and mortality. Controlling hyperinflammatory response is crucial in determining treatment direction. An important agent in providing this control is corticosteroids. This study aimed to determine whether dexamethasone and methylprednisolone, doses, administration time and duration in COVID-19 treatment are associated with improved treatment outcomes.
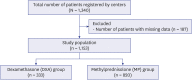
Methods: This retrospective multicenter study was conducted with participation of 6 healthcare centers which collected data by retrospectively examining files of 1,340 patients admitted to intensive care unit due to COVID-19 between March 2020 and September 2021, diagnosed with polymerase chain reaction (+) and/or clinically and radiologically.
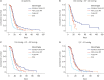
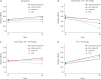
Results: Mortality in the pulse methylprednisolone group was statistically significantly higher than that in the other 3 groups. Mortality was higher in older patients with comorbidities such as hypertension, diabetes mellitus, chronic kidney failure, coronary artery disease, and dementia. Pulse and mini-pulse steroid doses were less effective than standard methylprednisolone and dexamethasone doses, pulse steroid doses being associated with high mortality. Standard-dose methylprednisolone and dexamethasone led to similar effects, but standard dose methylprednisolone was more effective in severe patients who required mechanical ventilation (MV). Infection development was related to steroid treatment duration, not cumulative steroid dose.
Conclusion: Corticosteroids are shown to be beneficial in critical COVID-19, but the role of early corticosteroids in mild COVID-19 patients remains unclear. The anti-inflammatory effects of corticosteroids may have a positive effect by reducing mortality in severe COVID-19 patients. Although dexamethasone was first used for this purpose, methylprednisolone was found to be as effective at standard doses. Methylprednisolone administered at standard doses was associated with greater PaO2/FiO2 ratios than dexamethasone, especially in the severe group requiring MV. High dose pulse steroid doses are closely associated with mortality and standard methylprednisolone dose is recommended.
Keywords: COVID-19; Dexamethasone; Intensive Care; Methylprednisolone; Secondary Infections.
© 2023 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

