Liraglutide pretreatment attenuates sepsis-induced acute lung injury
- PMID: 37489855
- PMCID: PMC10639010
- DOI: 10.1152/ajplung.00041.2023
Liraglutide pretreatment attenuates sepsis-induced acute lung injury
Abstract
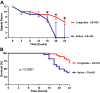
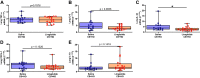
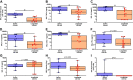
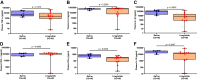
There are no effective targeted therapies to treat acute respiratory distress syndrome (ARDS). Recently, the commonly used diabetes and obesity medications, glucagon-like peptide-1 (GLP-1) receptor agonists, have been found to have anti-inflammatory properties. We, therefore, hypothesized that liraglutide pretreatment would attenuate murine sepsis-induced acute lung injury (ALI). We used a two-hit model of ALI (sepsis+hyperoxia). Sepsis was induced by intraperitoneal injection of cecal slurry (CS; 2.4 mg/g) or 5% dextrose (control) followed by hyperoxia [HO; fraction of inspired oxygen ([Formula: see text]) = 0.95] or room air (control; [Formula: see text] = 0.21). Mice were pretreated twice daily with subcutaneous injections of liraglutide (0.1 mg/kg) or saline for 3 days before initiation of CS+HO. At 24-h post CS+HO, physiological dysfunction was measured by weight loss, severity of illness score, and survival. Animals were euthanized, and bronchoalveolar lavage (BAL) fluid, lung, and spleen tissues were collected. Bacterial burden was assessed in the lung and spleen. Lung inflammation was assessed by BAL inflammatory cell numbers, cytokine concentrations, lung tissue myeloperoxidase activity, and cytokine expression. Disruption of the alveolar-capillary barrier was measured by lung wet-to-dry weight ratios, BAL protein, and epithelial injury markers (receptor for advanced glycation end products and sulfated glycosaminoglycans). Histological evidence of lung injury was quantified using a five-point score with four parameters: inflammation, edema, septal thickening, and red blood cells (RBCs) in the alveolar space. Compared with saline treatment, liraglutide improved sepsis-induced physiological dysfunction and reduced lung inflammation, alveolar-capillary barrier disruption, and lung injury. GLP-1 receptor activation may hold promise as a novel treatment strategy for sepsis-induced ARDS. Additional studies are needed to better elucidate its mechanism of action.NEW & NOTEWORTHY In this study, pretreatment with liraglutide, a commonly used diabetes medication and glucagon-like peptide-1 (GLP-1) receptor agonist, attenuated sepsis-induced acute lung injury in a two-hit mouse model (sepsis + hyperoxia). Septic mice who received the drug were less sick, lived longer, and displayed reduced lung inflammation, edema, and injury. These therapeutic effects were not dependent on weight loss. GLP-1 receptor activation may hold promise as a new treatment strategy for sepsis-induced acute respiratory distress syndrome.
Keywords: GLP-1 receptor activation; acute lung injury; liraglutide; lung edema; lung inflammation.
Conflict of interest statement
Julie Bastarache is an editor of
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

