Management of individuals with germline pathogenic/likely pathogenic variants in CHEK2: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG)
- PMID: 37490054
- PMCID: PMC10623578
- DOI: 10.1016/j.gim.2023.100870
Management of individuals with germline pathogenic/likely pathogenic variants in CHEK2: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG)
Abstract
Purpose: Although the role of CHEK2 germline pathogenic variants in cancer predisposition is well known, resources for managing CHEK2 heterozygotes in clinical practice are limited.
Methods: An international workgroup developed guidance on clinical management of CHEK2 heterozygotes informed by peer-reviewed publications from PubMed.
Results: Although CHEK2 is considered a moderate penetrance gene, cancer risks may be considered as a continuous variable, which are influenced by family history and other modifiers. Consequently, early cancer detection and prevention for CHEK2 heterozygotes should be guided by personalized risk estimates. Such estimates may result in both downgrading lifetime breast cancer risks to those similar to the general population or upgrading lifetime risk to a level at which CHEK2 heterozygotes are offered high-risk breast surveillance according to country-specific guidelines. Risk-reducing mastectomy should be guided by personalized risk estimates and shared decision making. Colorectal and prostate cancer surveillance should be considered based on assessment of family history. For CHEK2 heterozygotes who develop cancer, no specific targeted medical treatment is recommended at this time.
Conclusion: Systematic prospective data collection is needed to establish the spectrum of CHEK2-associated cancer risks and to determine yet-unanswered questions, such as the outcomes of surveillance, response to cancer treatment, and survival after cancer diagnosis.
Keywords: CHEK2; Cancer predisposition; Cancer risk; Cancer surveillance; Inherited cancer.
Copyright © 2023 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Funding and support listed here did not support development of this document unless included in the acknowledgments section. H.H. is supported by the Cancer Research CRUK Catalyst Award, CanGene-CanVar, and has served on advisory boards for AstraZeneca. R.S. is supported by the German Cancer Aid and the Federal Ministry of Education and Research (BMBF), Germany. M.T. is supported by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre. D.R.S. is supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute (NCI), Rockville, Maryland, and also performs contract clinical telehealth services for Genome Medical, Inc., in accordance with relevant NCI ethics policies. All other authors declare no conflicts of interest.
Figures
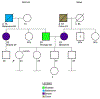
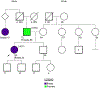
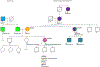


Comment in
-
Correspondence on "Management of individuals with germline pathogenic/likely pathogenic variants in CHEK2: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG)" by Hanson et al.Genet Med. 2024 Feb;26(2):101031. doi: 10.1016/j.gim.2023.101031. Epub 2023 Dec 29. Genet Med. 2024. PMID: 38156989 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

