Correlation of mucosal healing endpoints with long-term clinical and patient-reported outcomes in ulcerative colitis
- PMID: 37490069
- PMCID: PMC10522527
- DOI: 10.1007/s00535-023-02013-7
Correlation of mucosal healing endpoints with long-term clinical and patient-reported outcomes in ulcerative colitis
Abstract
Background: We evaluated the clinical relevance of achieving histologic endoscopic mucosal improvement (HEMI) and the more stringent target of histologic endoscopic mucosal remission (HEMR) in the phase 3 maintenance trial of upadacitinib for moderately to severely active ulcerative colitis.
Methods: Clinical and patient-reported outcomes were assessed in patients with clinical response after 8- or 16-week upadacitinib induction who received 52-week upadacitinib maintenance treatment. Cross-sectional and predictive analyses evaluated the relationship between HEMR or HEMI at Week 8/16 and Week 52, respectively, and outcomes at Week 52. Adjusted odds ratios (aOR) were derived from logistic regressions for patients achieving HEMR or HEMI without HEMR versus those not achieving HEMI.
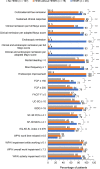
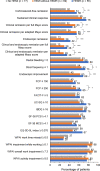
Results: Cross-sectional analyses showed that patients with HEMR had greater odds of achieving all clinical and patient-reported outcomes at Week 52 than those not achieving HEMI. In predictive analyses, patients with HEMR at Week 8/16 had significantly greater odds of achieving clinical remission (aOR = 3.6, p = 0.001) and endoscopic remission (aOR = 3.9, p < 0.001) at Week 52 than patients not achieving HEMI and HEMR. For patients achieving HEMI without HEMR, these odds were lower: clinical remission (aOR = 3.2, p < 0.001) and endoscopic remission (aOR = 2.4, p = 0.010). The odds of achieving clinically meaningful improvements in most patient-reported outcomes were directionally similar between HEMI and HEMR, but not statistically different to patients not achieving HEMI. No hospitalizations or surgeries were observed in patients with HEMR at Week 52.
Conclusions: Achievement of HEMR or HEMI is clinically relevant with HEMR being associated with greater likelihood of improvement in long-term clinical and patient-reported outcomes. https://www.
Clinicaltrials: gov NCT02819635.
Keywords: Histologic endoscopic mucosal improvement; Histologic endoscopic mucosal remission; Inflammatory bowel disease.
© 2023. Crown.
Conflict of interest statement
G Parkes received personal payments/honoraria/speaker fees and/or accepted travel grants or fellowships from AbbVie, Allergan, BMS, Celltrion, Ferring, Galapagos, Janssen, Napp, Takeda, and Tillotts; and is a Director and Shareholder in Ampersand Health. RC Ungaro served as an advisory board member or consultant for AbbVie, BMS, Janssen, Pfizer, and Takeda and has research grants from AbbVie, BI, and Pfizer. S Danese has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, BI, Celgene, Celltrion, Ferring, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor Pharma. MT Abreu has been a consultant or served on an advisory board for, served as a teacher, lecturer, or speaker for, and/or has received grants/research support from AbbVie, Alimentiv, Arena, BMS, Gilead, Imedex, Intellisphere, LLC (HSCG) HCP Live Institutional Perspectives in GI, Janssen, Lilly, Microba, Pfizer, Prime, Prometheus, Takeda, and UCB. E Arenson is an employee of Adelphi Values and provides scientific support services within the pharmaceutical and device development industry. W Zhou, D Ilo, FS Laroux, and Y Sanchez Gonzalez are employees of AbbVie and may own stock or stock options. H Deng is a research fellow and student at University of Illinois at Chicago. L Peyrin-Biroulet received personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; has stock options with CTMA.
Figures
Similar articles
-
Resolving Histological Inflammation in Ulcerative Colitis With Mirikizumab in the LUCENT Induction and Maintenance Trial Programmes.J Crohns Colitis. 2023 Oct 20;17(9):1457-1470. doi: 10.1093/ecco-jcc/jjad050. J Crohns Colitis. 2023. PMID: 37057827 Free PMC article. Clinical Trial.
-
Relationship Between Combined Histologic and Endoscopic Endpoints and Efficacy of Ustekinumab Treatment in Patients With Ulcerative Colitis.Gastroenterology. 2020 Dec;159(6):2052-2064. doi: 10.1053/j.gastro.2020.08.037. Epub 2020 Aug 25. Gastroenterology. 2020. PMID: 32853634 Clinical Trial.
-
Early Change in Epithelial Neutrophilic Infiltrate Predicts Long-Term Response to Biologics in Ulcerative Colitis.Clin Gastroenterol Hepatol. 2022 May;20(5):1095-1104.e9. doi: 10.1016/j.cgh.2021.07.005. Epub 2021 Jul 3. Clin Gastroenterol Hepatol. 2022. PMID: 34229037
-
Endoscopic mucosal healing and histologic remission in ulcerative colitis: a systematic literature review of clinical, quality-of-life and economic outcomes.Curr Med Res Opin. 2022 Sep;38(9):1531-1541. doi: 10.1080/03007995.2022.2081453. Epub 2022 Jun 11. Curr Med Res Opin. 2022. PMID: 35608153
-
Histologic assessments in ulcerative colitis: the evidence behind a new endpoint in clinical trials.Expert Rev Gastroenterol Hepatol. 2024 Jan-Feb;18(1-3):73-87. doi: 10.1080/17474124.2024.2326838. Epub 2024 Mar 21. Expert Rev Gastroenterol Hepatol. 2024. PMID: 38509826 Review.
Cited by
-
Disease clearance in ulcerative colitis: A new therapeutic target for the future.World J Gastroenterol. 2024 Apr 7;30(13):1801-1809. doi: 10.3748/wjg.v30.i13.1801. World J Gastroenterol. 2024. PMID: 38659483 Free PMC article. Review.
-
Multi-task deep learning framework for enhancing Mayo endoscopic score classification in ulcerative colitis.Digit Health. 2025 Jul 3;11:20552076251356396. doi: 10.1177/20552076251356396. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40621175 Free PMC article.
-
A Treat-to-Target Approach in IBD: Contemporary Real-World Perspectives from an International Survey.J Clin Med. 2025 Jan 21;14(3):667. doi: 10.3390/jcm14030667. J Clin Med. 2025. PMID: 39941338 Free PMC article.
-
Histologic and Endoscopic Findings Are Highly Correlated in a Prospective Cohort of Patients With Inflammatory Bowel Diseases.J Crohns Colitis. 2025 Jun 4;19(6):jjae141. doi: 10.1093/ecco-jcc/jjae141. J Crohns Colitis. 2025. PMID: 39739605 Free PMC article.
-
Disease Clearance in Ulcerative Colitis: A Narrative Review.United European Gastroenterol J. 2025 Jul;13(6):902-910. doi: 10.1002/ueg2.12714. Epub 2025 Apr 16. United European Gastroenterol J. 2025. PMID: 40237360 Free PMC article. Review.
References
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

