Real-World Outcomes of Trilaciclib Among Patients with Extensive-Stage Small Cell Lung Cancer Receiving Chemotherapy
- PMID: 37490258
- PMCID: PMC10499684
- DOI: 10.1007/s12325-023-02601-2
Real-World Outcomes of Trilaciclib Among Patients with Extensive-Stage Small Cell Lung Cancer Receiving Chemotherapy
Abstract
Introduction: Trilaciclib was recently approved in the USA for reducing chemotherapy-induced myelosuppression (CIM) among adults with extensive-stage small cell lung cancer (ES-SCLC) when administered prior to chemotherapy. There is limited understanding of real-world outcomes of trilaciclib.
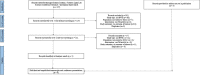
Methods: A comprehensive literature review was conducted using a keyword search in the MEDLINE, Embase, and conference abstracts. Additional studies were identified through communications with the authors of relevant studies. Published and unpublished real-world studies of trilaciclib- and comparable non-trilaciclib-treated patients with ES-SCLC were included. Evidence on myelosuppressive hematologic adverse events (HAEs), cytopenia-related healthcare utilization, and other reported outcomes (e.g., hospitalizations, dose reduction, and treatment delay) were synthesized. If feasible, outcomes were compared qualitatively between the trilaciclib and historical reference groups, and between first-line trilaciclib initiators and the overall trilaciclib population. Weighted averages were estimated for selected outcomes using sample size as the weight.
Results: The literature search identified five unique studies based on eight records-two included trilaciclib only, two non-trilaciclib only, and one both. In trilaciclib cohorts, the weighted average prevalence of grade ≥ 3 myelosuppressive HAEs in ≥ 1 lineage, ≥ 2 lineages, and all three lineages was 40.5%, 14.5%, and 7.5%, respectively. All rates were numerically lower compared to the historical non-trilaciclib cohorts (58.8%, 28.0%, 13.0% respectively). Cytopenia-related healthcare utilization was also lower in the trilaciclib cohorts. In general, first-line trilaciclib initiators had numerically lower myelosuppressive HAEs and cytopenia-related healthcare utilization than the overall trilaciclib patients.
Conclusions: The existing evidence suggests that trilaciclib may reduce single and multilineage grade ≥ 3 myelosuppressive HAEs and cytopenia-related healthcare utilization among patients with ES-SCLC in the real world. It is a promising new treatment for CIM prevention in ES-SCLC and may bring greater benefits to first-line trilaciclib initiators. Future studies are recommended to further evaluate the real-world effectiveness of trilaciclib.
Keywords: Chemotherapy-induced myelosuppression; Cytopenia; Extensive-stage small cell lung cancer; Real world; Supportive care; Trilaciclib.
© 2023. The Author(s).
Conflict of interest statement
L Lopez-Gonzalez was an employee of G1 Therapeutics, Inc. at the time of the study and is currently an employee of BioCryst Pharmaceuticals, Inc. H Huang is an employee of G1 Therapeutics, Inc. J Goldschmidt is an employee of the US Oncology Network, which received funding from G1 Therapeutics, Inc., for the studies included in this literature review. A Monnette, P Shi, D Venkatasetty, PR Conkling are employees of Ontada, which received funding from G1 Therapeutics, Inc., for the studies included in this literature review. L Hart, K Boykin, R Bailey, T Heritage, and L Gordan are employees of the Florida Cancer Specialists & Research Institute, which received funding from G1 Therapeutics, Inc., for the studies included in this literature review. J Scott and L Aton are employees of Integra Connect, which received funding from G1 Therapeutics, Inc., for the studies included in this literature review. A Ogbonnaya was an employee of Xcenda at the time of the study and is currently an employee of AbbVie. K Deyoung is an employee of Xcenda, which received funding from G1 Therapeutics, Inc., for the studies included in this literature review. Z Zhou and M Edwards are employees of Analysis Group, Inc., which received funding from G1 Therapeutics, Inc. for this literature review.
Figures


References
-
- US Food and Drug Administration. Atezolizumab product label. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761034s047lbl.pdf. Accessed 15 Mar 2023.
-
- US Food and Drug Administration. Durvalumab product label. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761069s033lbl.pdf. Accessed 15 Mar 2023.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

