Mesalazine granules promote disease clearance in patients with mild-to-moderate ulcerative colitis
- PMID: 37490352
- PMCID: PMC10576598
- DOI: 10.1002/ueg2.12435
Mesalazine granules promote disease clearance in patients with mild-to-moderate ulcerative colitis
Abstract
Background: Over the past decade, treatment targets for ulcerative colitis (UC) have become more stringent, incorporating multiple parameters. Recently, the concept of 'disease clearance'-defined as combined clinical, endoscopic, and histological remission-has been proposed as an ultimate endpoint in treating UC.
Objective: To determine the rates of disease clearance in patients with mild-to-moderate UC treated with different doses of mesalazine granules as induction therapy.
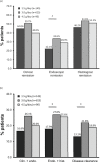
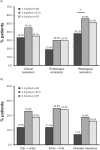
Methods: In a post hoc analysis, data were pooled from four randomised, active-controlled, phase 3 clinical trials in patients with mild-to-moderate UC receiving 8-week induction therapy with mesalazine granules at daily doses of 1.5, 3.0 or 4.5 g. Rates of clinical, endoscopic, and histological remission were determined using stringent criteria and used to calculate rates of the composite endpoints of clinical plus endoscopic remission, endoscopic plus histological remission, and disease clearance (clinical plus endoscopic plus histological remission).
Results: A total of 860 patients were included in the analysis. Among the total population, 20.0% achieved disease clearance with mesalazine granules: 13.1% in patients receiving 1.5 g mesalazine granules/day, 21.8% in those receiving 3.0 g/day and 18.9% in those receiving 4.5 g/day. Among patients with moderate UC, 16.8% achieved disease clearance: 7.1% with 1.5 g/day, 18.8% with 3.0 g/day and 16.2% with 4.5 g/day.
Conclusion: Disease clearance, proposed to be predictive of improved long-term outcomes, can be achieved in a clinically meaningful proportion of mild-to-moderate UC patients treated with mesalazine granules. A daily dose of 3.0 g appears optimal to reach this target.
Keywords: Inflammatory Bowel Disease; Ulcerative Colitis; clinical remission; disease clearance; endoscopic remission; mesalazine.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
These authors disclose the following: Wolfgang Kruis has received honoraria from Dr. Falk Pharma GmbH. Silke Meszaros, Sarah Wehrum, Roland Greinwald, Ralph Mueller and Tanju Nacak are employees of Dr. Falk Pharma GmbH. [Correction added on 09 August 2023, after first online publication: Sarah Wehrum’s disclosure has been added in this version.]
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

