Spectroscopically Orthogonal Spin Labels in Structural Biology at Physiological Temperatures
- PMID: 37490415
- PMCID: PMC10405217
- DOI: 10.1021/acs.jpcb.3c04497
Spectroscopically Orthogonal Spin Labels in Structural Biology at Physiological Temperatures
Abstract
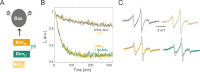
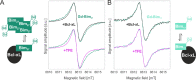
Electron paramagnetic resonance spectroscopy (EPR) is mostly used in structural biology in conjunction with pulsed dipolar spectroscopy (PDS) methods to monitor interspin distances in biomacromolecules at cryogenic temperatures both in vitro and in cells. In this context, spectroscopically orthogonal spin labels were shown to increase the information content that can be gained per sample. Here, we exploit the characteristic properties of gadolinium and nitroxide spin labels at physiological temperatures to study side chain dynamics via continuous wave (cw) EPR at X band, surface water dynamics via Overhauser dynamic nuclear polarization at X band and short-range distances via cw EPR at high fields. The presented approaches further increase the accessible information content on biomolecules tagged with orthogonal labels providing insights into molecular interactions and dynamic equilibria that are only revealed under physiological conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Lueders P.; Jeschke G.; Yulikov M. Double Electron–Electron Resonance Measured Between Gd3+ Ions and Nitroxide Radicals. J. Phys. Chem. Lett. 2011, 2, 604–609. 10.1021/jz200073h. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

