Fast protein folding is governed by memory-dependent friction
- PMID: 37490533
- PMCID: PMC10401029
- DOI: 10.1073/pnas.2220068120
Fast protein folding is governed by memory-dependent friction
Abstract
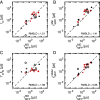
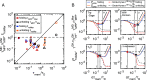
When described by a low-dimensional reaction coordinate, the folding rates of most proteins are determined by a subtle interplay between free-energy barriers, which separate folded and unfolded states, and friction. While it is commonplace to extract free-energy profiles from molecular trajectories, a direct evaluation of friction is far more elusive and typically relies on fits of measured reaction rates to memoryless reaction-rate theories. Here, using memory-kernel extraction methods founded on a generalized Langevin equation (GLE) formalism, we directly calculate the time-dependent friction acting on the fraction of native contacts reaction coordinate Q, evaluated for eight fast-folding proteins, taken from a published set of large-scale molecular dynamics protein simulations. Our results reveal that, across the diverse range of proteins represented in this dataset, friction is more influential than free-energy barriers in determining protein folding rates. We also show that proteins fold in a regime where the finite decay time of friction significantly reduces the folding times, in some instances by as much as a factor of 10, compared to predictions based on memoryless friction.
Keywords: generalized Langevin equation; molecular friction; non-Markovian dynamics; protein folding; reaction rate theory.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Bryngelson J. D., Wolynes P. G., Intermediates and barrier crossing in a random energy model (with applications to protein folding). J. Phys. Chem. 93, 6902–6915 (1989).
-
- Bryngelson J. D., Onuchic J. N., Socci N. D., Wolynes P. G., Funnels, pathways, and the energy landscape of protein folding: A synthesis. Proteins: Struct. 21 (1995). - PubMed
-
- Dill K. A., Chan H. S., From Levinthal to pathways to funnels. Nat. Struct. Biol. 4, 10–19 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

