Identification of a β-arrestin-biased negative allosteric modulator for the β2-adrenergic receptor
- PMID: 37490535
- PMCID: PMC10401000
- DOI: 10.1073/pnas.2302668120
Identification of a β-arrestin-biased negative allosteric modulator for the β2-adrenergic receptor
Abstract
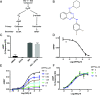
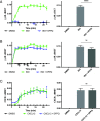
Catecholamine-stimulated β2-adrenergic receptor (β2AR) signaling via the canonical Gs-adenylyl cyclase-cAMP-PKA pathway regulates numerous physiological functions, including the therapeutic effects of exogenous β-agonists in the treatment of airway disease. β2AR signaling is tightly regulated by GRKs and β-arrestins, which together promote β2AR desensitization and internalization as well as downstream signaling, often antithetical to the canonical pathway. Thus, the ability to bias β2AR signaling toward the Gs pathway while avoiding β-arrestin-mediated effects may provide a strategy to improve the functional consequences of β2AR activation. Since attempts to develop Gs-biased agonists and allosteric modulators for the β2AR have been largely unsuccessful, here we screened small molecule libraries for allosteric modulators that selectively inhibit β-arrestin recruitment to the receptor. This screen identified several compounds that met this profile, and, of these, a difluorophenyl quinazoline (DFPQ) derivative was found to be a selective negative allosteric modulator of β-arrestin recruitment to the β2AR while having no effect on β2AR coupling to Gs. DFPQ effectively inhibits agonist-promoted phosphorylation and internalization of the β2AR and protects against the functional desensitization of β-agonist mediated regulation in cell and tissue models. The effects of DFPQ were also specific to the β2AR with minimal effects on the β1AR. Modeling, mutagenesis, and medicinal chemistry studies support DFPQ derivatives binding to an intracellular membrane-facing region of the β2AR, including residues within transmembrane domains 3 and 4 and intracellular loop 2. DFPQ thus represents a class of biased allosteric modulators that targets an allosteric site of the β2AR.
Keywords: G protein–coupled receptor; asthma; biased signaling; cell signaling; negative allosteric modulator.
Conflict of interest statement
A patent on the reported compounds was submitted by several of the authors (M.I., N.H., J.M.S., R.S.A., C.P.S., and J.L.B.) in 2022.
Figures





References
-
- Quirce S., Bobolea I., Barranco P., Emerging drugs for asthma. Expert Opin. Emerg. Drugs 17, 219–237 (2012). - PubMed
-
- Christopoulos A., Advances in G protein-coupled receptor allostery: From function to structure. Mol. Pharmacol. 86, 463–478 (2014). - PubMed
-
- Deshpande D. A., Penn R. B., Targeting G protein-coupled receptor signaling in asthma. Cell. Signal. 18, 2105–2120 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

