Artificial Sweeteners and Risk of Type 2 Diabetes in the Prospective NutriNet-Santé Cohort
- PMID: 37490630
- PMCID: PMC10465821
- DOI: 10.2337/dc23-0206
Artificial Sweeteners and Risk of Type 2 Diabetes in the Prospective NutriNet-Santé Cohort
Abstract
Objective: To study the relationships between artificial sweeteners, accounting for all dietary sources (total and by type of artificial sweetener) and risk of type 2 diabetes (T2D), in a large-scale prospective cohort.
Research design and methods: The analyses included 105,588 participants from the web-based NutriNet-Santé study (France, 2009-2022; mean age 42.5 ± 14.6 years, 79.2% women). Repeated 24-h dietary records, including brands and commercial names of industrial products, merged with qualitative and quantitative food additive composition data, enabled artificial sweetener intakes to be accurately assessed from all dietary sources. Associations between artificial sweeteners (total, aspartame, acesulfame potassium [K], and sucralose) and T2D were investigated using Cox proportional hazard models adjusted for potential confounders, including weight variation during follow-up.
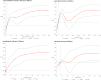
Results: During a median follow-up of 9.1 years (946,650 person-years, 972 incident T2D), compared with nonconsumers, higher consumers of artificial sweeteners (i.e., above the sex-specific medians of 16.4 mg/day in men and 18.5 mg/day in women) had higher risks of developing T2D (hazard ratio [HR] 1.69; 95% CI 1.45-1.97; P-trend <0.001). Positive associations were also observed for individual artificial sweeteners: aspartame (HR 1.63 [95% CI 1.38-1.93], P-trend <0.001), acesulfame-K (HR 1.70 [1.42-2.04], P-trend <0.001), and sucralose (HR 1.34 [1.07-1.69], P-trend = 0.013).
Conclusions: Potential for reverse causality cannot be eliminated; however, many sensitivity analyses were computed to limit this and other potential biases. These findings of positive associations between artificial sweetener intakes and increased T2D risk strengthen the evidence that these additives may not be safe sugar alternatives. This study provides important insights in the context of on-going reevaluation of artificial sweeteners by health authorities worldwide.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures


Comment in
-
Süßstoffe begünstigen Diabetes-Entwicklung.MMW Fortschr Med. 2024 Feb;166(3):28. doi: 10.1007/s15006-024-3649-1. MMW Fortschr Med. 2024. PMID: 38389002 German. No abstract available.
References
-
- Mooradian AD, Smith M, Tokuda M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: a narrative review. Clin Nutr ESPEN 2017;18:1–8 - PubMed
-
- U.S. Food and Drug Administration . Aspartame and Other Sweeteners in Food. 2018. Accessed 2 March 2018. Available from https://www.fda.gov/food/food-additives-petitions/additional-information...
-
- Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail. Ciqual Table de composition nutritionnelle des aliments. Accessed 8 February 2021. Available from https://ciqual.anses.fr/
-
- Rios-Leyvraz M, Montez J. Health effects of the use of non-sugar sweeteners: a systematic review and meta-analysis. Geneva: World Health Organization, 2022. Accessed 27 April 2022. Available from https://www.who.int/publications/i/item/9789240046429
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

