T cell reactivity to Bordetella pertussis is highly diverse regardless of childhood vaccination
- PMID: 37490913
- PMCID: PMC10528758
- DOI: 10.1016/j.chom.2023.06.015
T cell reactivity to Bordetella pertussis is highly diverse regardless of childhood vaccination
Abstract
The incidence of whooping cough due to Bordetella pertussis (BP) infections has increased recently. It is believed that the shift from whole-cell pertussis (wP) vaccines to acellular pertussis (aP) vaccines may be contributing to this rise. While T cells are key in controlling and preventing disease, nearly all knowledge relates to antigens in aP vaccines. A whole-genome mapping of human BP-specific CD4+ T cell responses was performed in healthy vaccinated adults and revealed unexpected broad reactivity to hundreds of antigens. The overall pattern and magnitude of T cell responses to aP and non-aP vaccine antigens are similar regardless of childhood vaccination, suggesting that asymptomatic infections drive the pattern of T cell reactivity in adults. Lastly, lack of Th1/Th2 polarization to non-aP vaccine antigens suggests these antigens have the potential to counteract aP vaccination Th2 bias. These findings enhance our insights into human T cell responses to BP and identify potential targets for next-generation pertussis vaccines.
Keywords: ORF; T cell; accelular vaccine; antigen; asymptomatic; childhood vaccination; epitope; infection; pertussis; polarization; whole-cell vaccine.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The La Jolla Institute for Immunology has filed for patent protection for various aspects of the BP epitope pools design.
Figures

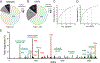

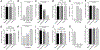


References
-
- CDC (2023). Pertusis (Whooping Cough).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

