An agonistic anti-signal regulatory protein α antibody for chronic inflammatory diseases
- PMID: 37490914
- PMCID: PMC10439247
- DOI: 10.1016/j.xcrm.2023.101130
An agonistic anti-signal regulatory protein α antibody for chronic inflammatory diseases
Abstract
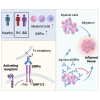
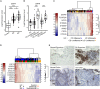
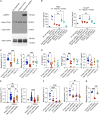
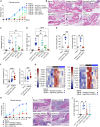
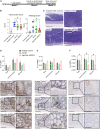
Signal regulatory protein (SIRPα) is an immune inhibitory receptor expressed by myeloid cells to inhibit immune cell phagocytosis, migration, and activation. Despite the progress of SIRPα and CD47 antagonist antibodies to promote anti-cancer immunity, it is not yet known whether SIRPα receptor agonism could restrain excessive autoimmune tissue inflammation. Here, we report that neutrophil- and monocyte-associated genes including SIRPA are increased in inflamed tissue biopsies from patients with rheumatoid arthritis and inflammatory bowel diseases, and elevated SIRPA is associated with treatment-refractory ulcerative colitis. We next identify an agonistic anti-SIRPα antibody that exhibits potent anti-inflammatory effects in reducing neutrophil and monocyte chemotaxis and tissue infiltration. In preclinical models of arthritis and colitis, anti-SIRPα agonistic antibody ameliorates autoimmune joint inflammation and inflammatory colitis by reducing neutrophils and monocytes in tissues. Our work provides a proof of concept for SIRPα receptor agonism for suppressing excessive innate immune activation and chronic inflammatory disease treatment.
Keywords: SIRPα; agonistic antibody; arthritis; autoimmune inflammation; colitis; monocyte; neutrophil.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors except D.A.F. are current or past employees of Genentech, a member of the Roche group, and may hold Roche stock or stock options.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

