REducing INFectiOns thRough Cardiac device Envelope: insight from real world data. The REINFORCE project
- PMID: 37490930
- PMCID: PMC10637307
- DOI: 10.1093/europace/euad224
REducing INFectiOns thRough Cardiac device Envelope: insight from real world data. The REINFORCE project
Abstract
Aims: Infections resulting from cardiac implantable electronic device (CIED) implantation are severely impacting on patients' and on health care systems. The use of TYRXTM absorbable antibiotic-eluting envelope has proven to decrease major CIED infections within 12 months of CIED surgery. The aim is to evaluate the impact of the envelope use on infection-related clinical events in a real-world contemporary patient population.
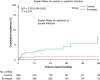
Methods and results: Data on patients undergoing CIED surgery were collected prospectively by participating centers of the One Hospital ClinicalService project. Patients were divided into two groups according to whether TYRXTM absorbable antibiotic-eluting envelope was used or not. Out of 1819 patients, 872 (47.9%) were implanted with an absorbable antibiotic-eluting envelope and included in the Envelope group and 947 (52.1%) patients who did not receive an envelope were included in the Control group. Compared to control, patients in the Envelope group had higher thrombo-embolic or hemorrhagic risk, higher BMI, lower LVEF and more comorbidities. During a mean follow-up of 1.4 years, the incidence of infection-related events was significantly higher in the control compared to the Envelope group (2.4% vs. 0.8%, P = 0.007). The five-year cumulative incidence of infection-related events was 8.1% in the control and 2.1% in the Envelope group (HR: 0.34, 95%CI: 0.14-0.80, P = 0.010).
Conclusion: In our analysis, the use of an absorbable antibiotic-eluting envelope in the general CIED population was associated with a lower risk of systemic and pocket infection.
Keywords: Antibiotic eluting envelope; CIED; Pacemaker; Pocket infection; Systemic infection.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: MZ received speaker’s fees from Abbott, Biotronik and Boston Scientific; GB reported small speaker fees from Bayer, Boehringer Ingelheim, Boston, Daiichi Sankyo, Janssen, and Sanofi outside of the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
Comment in
-
REINFORCING the clinical utility of an antibacterial envelope to prevent infection following a cardiac implantable electronic device implant procedure.Europace. 2023 Nov 2;25(11):euad260. doi: 10.1093/europace/euad260. Europace. 2023. PMID: 37672647 Free PMC article. No abstract available.
References
-
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MGet al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020;22:515–49. - PMC - PubMed
-
- Korantzopoulos P, Sideris S, Dilaveris P, Gatzoulis K, Goudevenos JA. Infection control in implantation of cardiac implantable electronic devices: current evidence, controversial points, and unresolved issues. Europace 2016;18:473–8. - PubMed
-
- Sławiński G, Lewicka E, Kempa M, Budrejko S, Racza G. Infections of cardiac implantable electronic devices: epidemiology, classification, treatment, and prognosis. Adv Clin Exp Med 2019;28:263–70. - PubMed
-
- Roder C, Gunjaca V, Otome O, Gwini SM, Athan E. Cost and outcomes of implantable cardiac electronic device infections in Victoria, Australia. Heart Lung Circ 2020;29:e140–6. - PubMed
-
- Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical