Computational Design of Potent and Selective d-Peptide Agonists of the Glucagon-like Peptide-2 Receptor
- PMID: 37491005
- PMCID: PMC10424673
- DOI: 10.1021/acs.jmedchem.3c00464
Computational Design of Potent and Selective d-Peptide Agonists of the Glucagon-like Peptide-2 Receptor
Abstract
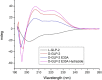
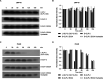
Here, we designed three d-GLP-2 agonists that activated the glucagon-like peptide-2 receptor (GLP-2R) cyclic adenosine monophosphate (cAMP) accumulation without stimulating the glucagon-like peptide-1 receptor (GLP-1R). All the d-GLP-2 agonists increased the protein kinase B phosphorylated (p-AKT) expression levels in a time- and concentration-dependent manner in vitro. The most effective d-GLP-2 analogue boosted the AKT phosphorylation 2.28 times more effectively compared to the native l-GLP-2. The enhancement in the p-AKT levels induced by the d-GLP-2 analogues could be explained by GLP-2R's more prolonged activation, given that the d-GLP-2 analogues induce a lower β-arrestin recruitment. The higher stability to protease degradation of our d-GLP-2 agonists helps us envision their potential applications in enhancing intestinal absorption and treating inflammatory bowel illness while lowering the high dosage required by the current treatments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor.J Biol Chem. 1999 Oct 22;274(43):30459-67. doi: 10.1074/jbc.274.43.30459. J Biol Chem. 1999. PMID: 10521425
-
Abolishing β-arrestin recruitment is necessary for the full metabolic benefits of G protein-biased glucagon-like peptide-1 receptor agonists.Diabetes Obes Metab. 2024 Jan;26(1):65-77. doi: 10.1111/dom.15288. Epub 2023 Oct 5. Diabetes Obes Metab. 2024. PMID: 37795639
-
Pharmacological characterization of mono-, dual- and tri-peptidic agonists at GIP and GLP-1 receptors.Biochem Pharmacol. 2020 Jul;177:114001. doi: 10.1016/j.bcp.2020.114001. Epub 2020 Apr 29. Biochem Pharmacol. 2020. PMID: 32360365
-
Glucagon-like peptide-1 receptor action in the vasculature.Peptides. 2019 Jan;111:26-32. doi: 10.1016/j.peptides.2018.09.002. Epub 2018 Sep 15. Peptides. 2019. PMID: 30227157 Review.
-
Regulation of intestinal lipid and lipoprotein metabolism by the proglucagon-derived peptides glucagon like peptide 1 and glucagon like peptide 2.Curr Opin Lipidol. 2018 Apr;29(2):95-103. doi: 10.1097/MOL.0000000000000495. Curr Opin Lipidol. 2018. PMID: 29432213 Free PMC article. Review.
Cited by
-
HelixDiff, a Score-Based Diffusion Model for Generating All-Atom α-Helical Structures.ACS Cent Sci. 2024 Apr 5;10(5):1001-1011. doi: 10.1021/acscentsci.3c01488. eCollection 2024 May 22. ACS Cent Sci. 2024. PMID: 38799672 Free PMC article.
-
Metabolic and Nutritional Issues after Lower Digestive Tract Surgery: The Important Role of the Dietitian in a Multidisciplinary Setting.Nutrients. 2024 Jan 12;16(2):246. doi: 10.3390/nu16020246. Nutrients. 2024. PMID: 38257141 Free PMC article. Review.
-
De novo design of D-peptide ligands: Application to influenza virus hemagglutinin.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2426554122. doi: 10.1073/pnas.2426554122. Epub 2025 Jun 27. Proc Natl Acad Sci U S A. 2025. PMID: 40577121
References
-
- Liang Y. L.; Khoshouei M.; Deganutti G.; Glukhova A.; Koole C.; Peat T. S.; Radjainia M.; Plitzko J. M.; Baumeister W.; Miller L. J.; Hay D. L.; Christopoulos A.; Reynolds C. A.; Wootten D.; Sexton P. M. Cryo-Em Structure of the Active, G(S)-Protein Complexed, Human Cgrp Receptor. Nature 2018, 561, 492–497. 10.1038/s41586-018-0535-y. - DOI - PMC - PubMed
-
- Liang Y. L.; Khoshouei M.; Glukhova A.; Furness S. G. B.; Zhao P.; Clydesdale L.; Koole C.; Truong T. T.; Thal D. M.; Lei S.; Radjainia M.; Danev R.; Baumeister W.; Wang M. W.; Miller L. J.; Christopoulos A.; Sexton P. M.; Wootten D. Phase-Plate Cryo-Em Structure of a Biased Agonist-Bound Human Glp-1 Receptor-Gs Complex. Nature 2018, 555, 121–125. 10.1038/nature25773. - DOI - PubMed
-
- Ma S.; Shen Q.; Zhao L. H.; Mao C.; Zhou X. E.; Shen D. D.; de Waal P. W.; Bi P.; Li C.; Jiang Y.; Wang M. W.; Sexton P. M.; Wootten D.; Melcher K.; Zhang Y.; Xu H. E. Molecular Basis for Hormone Recognition and Activation of Corticotropin-Releasing Factor Receptors. Mol. Cell 2020, 77, 669–680.e4. 10.1016/j.molcel.2020.01.013. - DOI - PubMed
-
- Qiao A.; Han S.; Li X.; Li Z.; Zhao P.; Dai A.; Chang R.; Tai L.; Tan Q.; Chu X.; Ma L.; Thorsen T. S.; Reedtz-Runge S.; Yang D.; Wang M. W.; Sexton P. M.; Wootten D.; Sun F.; Zhao Q.; Wu B. Structural Basis of G(S) and G(I) Recognition by the Human Glucagon Receptor. Science 2020, 367, 1346–1352. 10.1126/science.aaz5346. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

