Quantifying the available capacity and resource needs for provision of CAR-T therapies in the National Health Service in Spain: a survey-based study
- PMID: 37491085
- PMCID: PMC10373688
- DOI: 10.1136/bmjopen-2022-071371
Quantifying the available capacity and resource needs for provision of CAR-T therapies in the National Health Service in Spain: a survey-based study
Abstract
Objectives: To estimate the readiness of Spanish National Health Service (NHS) hospitals to provide chimeric antigen receptor T cell (CAR-T), and to identify and quantify the different resources needed to provide CAR-T considering three scenarios defined by 10, 25 and 50 patients per centre per year.
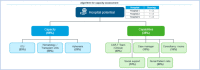
Design: Targeted literature review and quantitative study using a questionnaire and telephone interviews. An algorithm was created to determine hospitals' readiness based on their capacity and capability. All the requirements for quantification were assessed and validated by the steering committee, formed by members of the Spanish Group of Haematopoietic Transplantation and Cell Therapy. A weighting system (from 0 to 1) was established for capability quantification. For resources quantification, a scoring system was established, with 0 points representing the minimum and 3 points the maximum of additional resources that a hospital indicated necessary.
Setting: 40 Spanish hospital centres that perform allogeneic haematopoietic stem cell transplantation were invited to complete the questionnaire for capacity quantification, 28 of which provided valid responses. Nine hospitals participated in the interviews for resource quantification, eight of which had previously been designated by the Ministry of Health (MoH) to provide CAR-T.
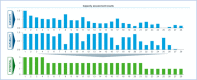
Outcome measure: Current capacity of NHS Spanish sites to administer CAR-T under different theoretical scenarios with varying numbers of procedures, and the potential healthcare resources that would be needed to realise the theoretical capacity requirements.
Results: Four hospitals were optimally ready, 17 were somewhat ready and 7 were not ready. The actual extrapolated capacity of the currently designated MoH CAR-T sites would allow treatment of approximately 250 patients per year. Regarding healthcare resource needs, the numbers of haematologists, nurses and beds were the most important limiting factors, and those requiring further growth as patient numbers increased.
Conclusions: Increasing the number of CAR-T-qualified centres and/or increasing resources in the current designated sites are two potential strategies that should be considered to treat CAR-T-eligible patients in Spain.
Keywords: haematology; leukaemia; lymphoma; organisation of health services; therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CS declares having received honoraria from BMS, Kite/Gilead, MSD, Pfizer, Jazz Pharmaceuticals, Novartis and Pierre Fabre. PC-R declares having received honoraria from participation in advisory boards from Alexion, Janssen and Gilead. PB-S declares having received honoraria from Allogene, Amgen, BMS, Kite/Gilead, Incyte, Jazz Pharmaceuticals, Miltenyi Biomedicine, Nektar Novartis and Pierre Fabre. VO declares having received honoraria from Kite, Celgene-BMS, Novartis, Miltenyi and Janssen. ACC declares having received honoraria from Gilead and Novartis. JM declares having received honoraria from AbbVie, Sanofi, BMS, Janssen, Gilead, Novartis and Roche.
Figures

References
-
- Peters C, Schrappe M, von Stackelberg A, et al. . Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors-the ALL-SCT-BFM-2003 trial. J Clin Oncol 2015;33:1265–74. 10.1200/JCO.2014.58.9747 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
