What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study's patient-level data with fidelity to the original research protocol
- PMID: 37491091
- PMCID: PMC10373710
- DOI: 10.1136/bmjopen-2022-063095
What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study's patient-level data with fidelity to the original research protocol
Abstract
Objective: Reanalyse the patient-level data set of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study with fidelity to the original research protocol and related publications.
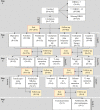
Design: The study was open label and semirandomised examining the effectiveness of up to four optimised and increasingly aggressive, antidepressant therapies in depressed adults. Patients who failed to gain adequate relief from their level 1 trial on the SSRI citalopram could receive up to three additional treatment trials in levels 2-4.
Setting: 41 North American psychiatry and primary care treatment centres.
Participants: 4041 adults screened positive for major depressive disorder. In contrast to most clinical trials, STAR*D enrolled patients seeking care (vs recruited) and included patients with a wide range of common comorbid medical and psychiatric conditions to enhance the generalisability of findings to real-world clinical practice.
Interventions: STAR*D evaluated the relative effectiveness of 13 antidepressants therapies in treatment levels 2-4 for depressed patients who failed to gain adequate benefit from their level 1 medication trial.
Main outcome measures: According to the STAR*D protocol, the primary outcome was remission, defined as a score <8 on the blinded Hamilton Rating Scale for Depression (HRSD). Response was a secondary outcome defined as ≥50% reduction in HRSD scores. STAR*D's protocol specifically excluded all non-blinded clinic-administered assessments from use as research outcome measures.
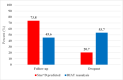
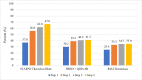
Results: STAR*D investigators did not use the protocol-stipulated HRSD to report cumulative remission and response rates in their summary article and instead used a non-blinded clinic-administered assessment. This inflated their report of outcomes, as did their inclusion of 99 patients who scored as remitted on the HRSD at study outset as well as 125 who scored as remitted when initiating their next-level treatment. These patients should have been excluded from data analysis. In contrast to the STAR*D-reported 67% cumulative remission rate after up to four antidepressant treatment trials, the rate was 35.0% when using the protocol-stipulated HRSD and inclusion in data analysis criteria.
Conclusion: STAR*D's cumulative remission rate was approximately half of that reported.
Keywords: Adult psychiatry; CLINICAL PHARMACOLOGY; Depression & mood disorders.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
The STAR*D Data Remain Strong: Reply to Pigott et al.Am J Psychiatry. 2023 Dec 1;180(12):919-920. doi: 10.1176/appi.ajp.20230869. Am J Psychiatry. 2023. PMID: 38037409 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
