The role of mitochondrial genes on nuclear gene expression in neovascular age related macular degeneration: analysis of nuclear VEGF gene expression after ranibizumab treatment in cytoplasmic hybrid retinal pigment epithelial cell lines correlated with clinical evolution
- PMID: 37491310
- PMCID: PMC10367366
- DOI: 10.1186/s40942-023-00476-7
The role of mitochondrial genes on nuclear gene expression in neovascular age related macular degeneration: analysis of nuclear VEGF gene expression after ranibizumab treatment in cytoplasmic hybrid retinal pigment epithelial cell lines correlated with clinical evolution
Abstract
Purpose: The present study tests the hypothesis that mitochondrial genes have retrograde signaling capacity that influences the expression of nuclear genes related to angiogenesis pathways. Cytoplasmic hybrid (cybrid) in vitro cell lines with patient specific mitochondria inserted into an immortalized retinal pigment epithelial cell line (ARPE-19) were used to test this hypothesis. This type of analysis can provide important information to identify the optimal regimen of anti-VEGF treatment, personalizing age-related macular degeneration (AMD) therapies.
Methods: Mitochondria deficient ARPE-19 cells (Rho0) were fused with AMD donor's platelets to create individual cybrid cell lines containing mitochondria from patients with phenotypic AMD disease and nuclear DNA from the immortalized RPE cell line. The cybrids were treated with Ranibizumab (Lucentis, Genentech, San Francisco, CA), at 4 different concentrations for 24 h, and subsequently the levels of reactive oxygen species (ROS), gene expression for VEGF-A, hypoxia-inducible factor 1-alpha (HIF1-a) and manganese superoxide dismutase (SOD2) were measured. The clinical evolution of the two AMD-donors were correlated with the molecular findings found in their 'personalized' cybrids.
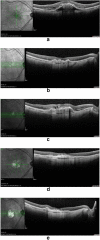
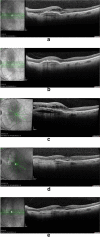
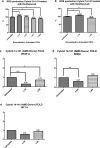
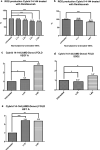
Results: Cybrids from Patient-01 showed down-regulation of gene expression of VEGF-A and HIF-1a at both 1X and 4X Ranibizumab concentrations. Patient-01 AMD cybrid cultures had an increase in the ROS levels at 1X (P = 0.0317), no changes at 2X (P = 0.8350) and a decrease at 4X (P = 0.0015) and 10X (P = 0.0011) of Ranibizumab. Clinically, Patient-01 responded to anti-VEGF therapy but eventually developed geographic atrophy. Patient-02 cybrids demonstrated up-regulation of gene expression of VEGF-A and HIF-1a at Ranibizumab 1X and 4X concentrations. There was decreased ROS levels with Ranibizumab 1X (P = 0.1606), 2X (P = 0.0388), 4X (P = 0.0010) and 10X (P = < 0.0001). Clinically, Patient-02 presented with a neovascular lesion associated with a prominent production of intraretinal fluid in clinical follow-up requiring regular and repeated intravitreal injections of Ranibizumab with recurrent subretinal fluid.
Conclusions: Our cybrid model has the potential to help personalize the treatment regimen with anti-VEGF drugs in patients with neovascular AMD. Further investigation is needed to better understand the role that the mitochondria play in the cellular response to anti-VEGF drugs. Future studies that focus on this model have the potential to help personalize anti-VEGF treatment.
© 2023. The Author(s).
Conflict of interest statement
Rodrigo Donato Costa: No competing interests Farid José Thomaz Neto: No competing interests M. Tarek Moustafa: No competing interests Shari R. Atilano: No competing interests Marilyn Chwa: No competing interests Javier Cáceres-del-Carpi: No competing interests Mohamed Hamid Mohamed: No competing interests M. Cristina Kenney: No competing interests Baruch D. Kuppermann: Clinical Research: Allegro Ophthalmics, Allergan, Genentech Inc, Ionis, IVERIC Bio, Novartis Pharmaceuticals, Regeneron Pharmaceuticals Inc, RegenXBio Consultant: Allegro Ophthalmics, Allergan, Aviceda Therapeutics, Clearside, EyeBio, Eyedaptic, Genentech Inc, Glaukos Corporation, InflammX Therapeutics, IVERIC Bio, jCyte, Novartis Pharmaceuticals, Regeneron Pharmaceuticals Inc, ReVana Therapeutics, Ripple Therapeutics, Theravance Biopharma Speaker Bureau: Allergan, Genentech.
Figures
Similar articles
-
Differential effects of risuteganib and bevacizumab on AMD cybrid cells.Exp Eye Res. 2021 Feb;203:108287. doi: 10.1016/j.exer.2020.108287. Epub 2020 Oct 16. Exp Eye Res. 2021. PMID: 33075294 Free PMC article.
-
Effects of bevacizumab, ranibizumab, and aflibercept on phagocytic properties in human RPE cybrids with AMD versus normal mitochondria.Exp Eye Res. 2018 Dec;177:112-116. doi: 10.1016/j.exer.2018.07.025. Epub 2018 Jul 30. Exp Eye Res. 2018. PMID: 30071215 Free PMC article.
-
Differential mitochondrial and cellular responses between H vs. J mtDNA haplogroup-containing human RPE transmitochondrial cybrid cells.Exp Eye Res. 2022 Jun;219:109013. doi: 10.1016/j.exer.2022.109013. Epub 2022 Mar 10. Exp Eye Res. 2022. PMID: 35283109 Free PMC article.
-
Defining response to anti-VEGF therapies in neovascular AMD.Eye (Lond). 2015 Jun;29(6):721-31. doi: 10.1038/eye.2015.48. Epub 2015 Apr 17. Eye (Lond). 2015. PMID: 25882328 Free PMC article. Review.
-
Pipeline therapies for neovascular age related macular degeneration.Int J Retina Vitreous. 2021 Oct 1;7(1):55. doi: 10.1186/s40942-021-00325-5. Int J Retina Vitreous. 2021. PMID: 34598731 Free PMC article. Review.
Cited by
-
Manganese exposure and its U-shaped relationship with diabetic retinopathy: analysis of NHANES 2011-2020.Front Nutr. 2025 Jun 19;12:1619751. doi: 10.3389/fnut.2025.1619751. eCollection 2025. Front Nutr. 2025. PMID: 40612319 Free PMC article.
References
-
- Emerson GG, Francis PJ, Wilson D, Garner A. Vascular diseases, Chapter 67. In: Klintworth G, Garner A, Editors. Pathophysiology of ocular diseases, 3rd edn. CRC Press; 2008.
-
- Kenney MC, Hertzog D, Chak G, Atilano SR, Khatibi N, Soe K, Nobe A, Yang E, Chwa M, Zhu F, Memarzadeh M, King J, Small K, Nesburn AB, Boyer DS, Udar N. Mitochondrial DNA haplogroups confer differences in risk for age-related macular degeneration: a case control study. BMC Medical Genetics. 2013;9;14(1):4. - PMC - PubMed

