Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression
- PMID: 37491321
- PMCID: PMC10368710
- DOI: 10.1038/s41392-023-01497-2
Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression
Abstract
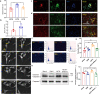
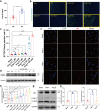
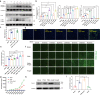
Atrial fibrillation (AF) is a frequent arrhythmia associated with cardiovascular morbidity and mortality. Neutrophil extracellular traps (NETs) are DNA fragments with cytoplasm proteins released from neutrophils, which are involved in various cardiovascular diseases. To elucidate the role of NETs in AF, we investigated the effect of NETs on AF progression and the secretion of NETs in AF. Results showed that: NETs induced the autophagic apoptosis of cardiomyocytes, and NETs also led to mitochondrial injury by promoting mitochondrial depolarization and ROS production. Ongoing tachy-pacing led to the structural loss of cardiomyocytes and provided potent stimuli to induce NETs secretion from neutrophils. In the meanwhile, increased Ang II in AF facilitated NETs formation through the upregulation of AKT phosphorylation, while it could not directly initiate NETosis as the autophagy was not induced. In vivo, DNase I was administrated to abrogate NETs formation, and AF-related fibrosis was ameliorated as expected. Correspondingly, the duration of the induced AF was reduced. Our study addresses the formation mechanism of NETs in AF and demonstrates the lethal effects of NETs on cardiomyocytes through the induction of mitochondrial injury and autophagic cell death, which comprehensively describes the positive feedback comprised of NETs and stimuli secreted by cardiomyocytes that sustains the progression of AF and AF related fibrosis.
© 2023. The Author(s).
Conflict of interest statement
Dr Yingqiang Guo and other authors have no financial or personal relationship with individuals or institutions that would inappropriately influence this work.
Figures



References
-
- Zhou ZQ, et al. [An epidemiological survey of atrial fibrillation in China] Zhonghua Nei Ke Za Zhi. 2004;43:491–494. - PubMed
-
- January CT, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

