Non-viral gene delivery to human mesenchymal stem cells: a practical guide towards cell engineering
- PMID: 37491322
- PMCID: PMC10369726
- DOI: 10.1186/s13036-023-00363-7
Non-viral gene delivery to human mesenchymal stem cells: a practical guide towards cell engineering
Abstract
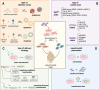
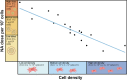
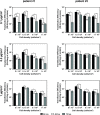
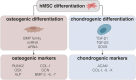
In recent decades, human mesenchymal stem cells (hMSCs) have gained momentum in the field of cell therapy for treating cartilage and bone injuries. Despite the tri-lineage multipotency, proliferative properties, and potent immunomodulatory effects of hMSCs, their clinical potential is hindered by donor variations, limiting their use in medical settings. To address this challenge, gene delivery technologies have emerged as a promising approach to modulate the phenotype and commitment of hMSCs towards specific cell lineages, thereby enhancing osteochondral repair strategies. This review provides a comprehensive overview of current non-viral gene delivery approaches used to engineer MSCs, highlighting key factors such as the choice of nucleic acid or delivery vector, transfection strategies, and experimental parameters. Additionally, it outlines various protocols and methods for qualitative and quantitative evaluation of their therapeutic potential as a delivery system in osteochondral regenerative applications. In summary, this technical review offers a practical guide for optimizing non-viral systems in osteochondral regenerative approaches. hMSCs constitute a key target population for gene therapy techniques. Nevertheless, there is a long way to go for their translation into clinical treatments. In this review, we remind the most relevant transfection conditions to be optimized, such as the type of nucleic acid or delivery vector, the transfection strategy, and the experimental parameters to accurately evaluate a delivery system. This survey provides a practical guide to optimizing non-viral systems for osteochondral regenerative approaches.
Keywords: Mesenchymal stem cells; Non-viral gene delivery vectors; Osteochondral repair; Regenerative medicine; Tissue engineering.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Treatment of osteochondral defects in the rabbit's knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots.J Vis Exp. 2013 May 21;(75):e4423. doi: 10.3791/4423. J Vis Exp. 2013. PMID: 23728213 Free PMC article.
-
Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration.Acta Biomater. 2017 Nov;63:210-226. doi: 10.1016/j.actbio.2017.09.008. Epub 2017 Sep 9. Acta Biomater. 2017. PMID: 28899816
-
PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency.Acta Biomater. 2015 Nov;27:42-52. doi: 10.1016/j.actbio.2015.08.046. Epub 2015 Aug 28. Acta Biomater. 2015. PMID: 26320543
-
Innovative nonviral gene delivery strategies for engineering human mesenchymal stem cell phenotypes toward clinical applications.Curr Opin Biotechnol. 2022 Dec;78:102819. doi: 10.1016/j.copbio.2022.102819. Epub 2022 Oct 21. Curr Opin Biotechnol. 2022. PMID: 36274497 Free PMC article. Review.
-
Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage.Biomaterials. 2016 Oct;104:223-37. doi: 10.1016/j.biomaterials.2016.07.017. Epub 2016 Jul 21. Biomaterials. 2016. PMID: 27467418 Review.
Cited by
-
Mesenchymal stem cells and their extracellular vesicles: new therapies for cartilage repair.Front Bioeng Biotechnol. 2025 Apr 24;13:1591400. doi: 10.3389/fbioe.2025.1591400. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40343207 Free PMC article. Review.
-
Cell macroencapsulation devices in contemporary research: A systematic review.Regen Ther. 2025 Jun 9;30:144-156. doi: 10.1016/j.reth.2025.05.013. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40547419 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

