Clinical Value of Longitudinal Serum Neurofilament Light Chain in Prodromal Genetic Frontotemporal Dementia
- PMID: 37491327
- PMCID: PMC10491440
- DOI: 10.1212/WNL.0000000000207581
Clinical Value of Longitudinal Serum Neurofilament Light Chain in Prodromal Genetic Frontotemporal Dementia
Abstract
Background and objectives: Elevated serum neurofilament light chain (NfL) is used to identify carriers of genetic frontotemporal dementia (FTD) pathogenic variants approaching prodromal conversion. Yet, the magnitude and timeline of NfL increase are still unclear. Here, we investigated the predictive and early diagnostic value of longitudinal serum NfL for the prodromal conversion in genetic FTD.
Methods: In a longitudinal observational cohort study of genetic FTD pathogenic variant carriers, we examined the diagnostic accuracy and conversion risk associated with cross-sectional and longitudinal NfL. Time periods relative to prodromal conversion (>3, 3-1.5, 1.5-0 years before; 0-1.5 years after) were compared with values of participants who did not convert. Next, we modeled longitudinal NfL and MRI volume trajectories to determine their timeline.
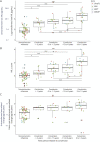
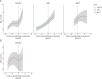
Results: We included 21 participants who converted (5 chromosome 9 open-reading frame 72 [C9orf72], 10 progranulin [GRN], 5 microtubule-associated protein tau [MAPT], and 1 TAR DNA-binding protein [TARDBP]) and 61 who did not (20 C9orf72, 30 GRN, and 11 MAPT). Participants who converted had higher NfL levels at all examined periods before prodromal conversion (median values 14.0-18.2 pg/mL; betas = 0.4-0.7, standard error [SE] = 0.1, p < 0.046) than those who did not (6.5 pg/mL) and showed further increase 0-1.5 years after conversion (28.4 pg/mL; beta = 1.0, SE = 0.1, p < 0.001). Annualized longitudinal NfL change was only significantly higher in participants who converted (vs. participants who did not) 0-1.5 years after conversion (beta = 1.2, SE = 0.3, p = 0.001). Diagnostic accuracy of cross-sectional NfL for prodromal conversion (vs. nonconversion) was good-to-excellent at time periods before conversion (area under the curve range: 0.72-0.92), improved 0-1.5 years after conversion (0.94-0.97), and outperformed annualized longitudinal change (0.76-0.84). NfL increase in participants who converted occurred earlier than frontotemporal MRI volume change and differed by genetic group and clinical phenotypes. Higher NfL corresponded to increased conversion risk (hazard ratio: cross-sectional = 6.7 [95% CI 3.3-13.7]; longitudinal = 13.0 [95% CI 4.0-42.8]; p < 0.001), but conversion-free follow-up time varied greatly across participants.
Discussion: NfL increase discriminates individuals who convert to prodromal FTD from those who do not, preceding significant frontotemporal MRI volume loss. However, NfL alone is limited in predicting the exact timing of prodromal conversion. NfL levels also vary depending on underlying variant-carrying genes and clinical phenotypes. These findings help to guide participant recruitment for clinical trials targeting prodromal genetic FTD.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



Comment in
-
Neurofilament marks prodrome onset in FTD.Nat Rev Neurol. 2023 Sep;19(9):508. doi: 10.1038/s41582-023-00860-9. Nat Rev Neurol. 2023. PMID: 37553391 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
