Epigenetic silencing of HTATIP2 in glioblastoma contributes to treatment resistance by enhancing nuclear translocation of the DNA repair protein MPG
- PMID: 37491696
- PMCID: PMC10483604
- DOI: 10.1002/1878-0261.13494
Epigenetic silencing of HTATIP2 in glioblastoma contributes to treatment resistance by enhancing nuclear translocation of the DNA repair protein MPG
Abstract
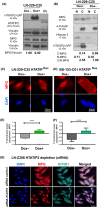
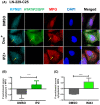
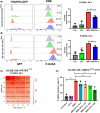
Glioblastoma, the most malignant brain tumor in adults, exhibits characteristic patterns of epigenetic alterations that await elucidation. The DNA methylome of glioblastoma revealed recurrent epigenetic silencing of HTATIP2, which encodes a negative regulator of importin β-mediated cytoplasmic-nuclear protein translocation. Its deregulation may thus alter the functionality of cancer-relevant nuclear proteins, such as the base excision repair (BER) enzyme N-methylpurine DNA glycosylase (MPG), which has been associated with treatment resistance in GBM. We found that induction of HTATIP2 expression in GBM cells leads to a significant shift of predominantly nuclear to cytoplasmic MPG, whereas depletion of endogenous HTATIP2 results in enhanced nuclear MPG localization. Reduced nuclear MPG localization, prompted by HTATIP2 expression or by depletion of MPG, yielded less phosphorylated-H2AX-positive cells upon treatment with an alkylating agent. This suggested reduced MPG-mediated formation of apurinic/apyrimidinic sites, leaving behind unrepaired DNA lesions, reflecting a reduced capacity of BER in response to the alkylating agent. Epigenetic silencing of HTATIP2 may thus increase nuclear localization of MPG, thereby enhancing the capacity of the glioblastoma cells to repair treatment-related lesions and contributing to treatment resistance.
Keywords: DNA damage repair; GBM; epigenetic silencing; nuclear-cytoplasmic translocation; treatment resistance.
© 2023 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
ATM regulates 3-methylpurine-DNA glycosylase and promotes therapeutic resistance to alkylating agents.Cancer Discov. 2014 Oct;4(10):1198-213. doi: 10.1158/2159-8290.CD-14-0157. Epub 2014 Aug 6. Cancer Discov. 2014. PMID: 25100205 Free PMC article.
-
N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide.Neuro Oncol. 2011 May;13(5):471-86. doi: 10.1093/neuonc/nor011. Epub 2011 Mar 3. Neuro Oncol. 2011. PMID: 21377995 Free PMC article.
-
C1q/TNF-related peptide 8 (CTRP8) promotes temozolomide resistance in human glioblastoma.Mol Oncol. 2018 Sep;12(9):1464-1479. doi: 10.1002/1878-0261.12349. Epub 2018 Aug 2. Mol Oncol. 2018. PMID: 29949238 Free PMC article.
-
UV-DDB as a General Sensor of DNA Damage in Chromatin: Multifaceted Approaches to Assess Its Direct Role in Base Excision Repair.Int J Mol Sci. 2023 Jun 15;24(12):10168. doi: 10.3390/ijms241210168. Int J Mol Sci. 2023. PMID: 37373320 Free PMC article. Review.
-
BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis.Prog Nucleic Acid Res Mol Biol. 2001;68:41-54. doi: 10.1016/s0079-6603(01)68088-7. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554312 Review.
Cited by
-
Unraveling anoikis in glioblastoma: insights from single-cell sequencing and prognostic modeling.Cancer Cell Int. 2025 Mar 26;25(1):116. doi: 10.1186/s12935-025-03752-8. Cancer Cell Int. 2025. PMID: 40140848 Free PMC article.
-
ER stress-driven unfolded protein response fuels aging-related tumor aggressiveness in gliomas.Front Mol Biosci. 2025 Jul 11;12:1640038. doi: 10.3389/fmolb.2025.1640038. eCollection 2025. Front Mol Biosci. 2025. PMID: 40718795 Free PMC article.
-
PRIM2: A Marker of MYC-driven Hyper-proliferation, Disease Progression, Tumor Aggressiveness and Poor Survival in Glioma Patients.Cancer Genomics Proteomics. 2024 Mar-Apr;21(2):186-202. doi: 10.21873/cgp.20440. Cancer Genomics Proteomics. 2024. PMID: 38423596 Free PMC article.
-
Characterization of NOD-like receptor-based molecular heterogeneity in glioma and its association with immune micro-environment and metabolism reprogramming.Front Immunol. 2025 Jan 15;15:1498583. doi: 10.3389/fimmu.2024.1498583. eCollection 2024. Front Immunol. 2025. PMID: 39882240 Free PMC article.
-
Glioblastoma multiforme: an updated overview of temozolomide resistance mechanisms and strategies to overcome resistance.Discov Oncol. 2025 May 12;16(1):731. doi: 10.1007/s12672-025-02567-3. Discov Oncol. 2025. PMID: 40353925 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol. 2009;10:459–466. 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. 10.1158/2159-8290.CD-21-1059 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

