Lysosome-dependent FOXA1 ubiquitination contributes to luminal lineage of advanced prostate cancer
- PMID: 37491794
- PMCID: PMC10552895
- DOI: 10.1002/1878-0261.13497
Lysosome-dependent FOXA1 ubiquitination contributes to luminal lineage of advanced prostate cancer
Abstract
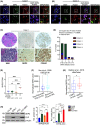
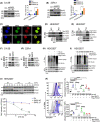
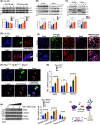
Changes in FOXA1 (forkhead box protein A1) protein levels are well associated with prostate cancer (PCa) progression. Unfortunately, direct targeting of FOXA1 in progressive PCa remains challenging due to variations in FOXA1 protein levels, increased FOXA1 mutations at different stages of PCa, and elusive post-translational FOXA1 regulating mechanisms. Here, we show that SKP2 (S-phase kinase-associated protein 2) catalyzes K6- and K29-linked polyubiquitination of FOXA1 for lysosomal-dependent degradation. Our data indicate increased SKP2:FOXA1 protein ratios in stage IV human PCa compared to stages I-III, together with a strong inverse correlation (r = -0.9659) between SKP2 and FOXA1 levels, suggesting that SKP2-FOXA1 protein interactions play a significant role in PCa progression. Prostate tumors of Pten/Trp53 mice displayed increased Skp2-Foxa1-Pcna signaling and colocalization, whereas disruption of the Skp2-Foxa1 interplay in Pten/Trp53/Skp2 triple-null mice demonstrated decreased Pcna levels and increased expression of Foxa1 and luminal positive cells. Treatment of xenograft mice with the SKP2 inhibitor SZL P1-41 decreased tumor proliferation, SKP2:FOXA1 ratios, and colocalization. Thus, our results highlight the significance of the SKP2-FOXA1 interplay on the luminal lineage in PCa and the potential of therapeutically targeting FOXA1 through SKP2 to improve PCa control.
Keywords: FOXA1; SKP2; luminal lineage; prostate cancer; ubiquitination.
© 2023 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73(2023):17–48. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen‐independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

