Self-Assembly of Insulin-Derived Chimeric Peptides into Two-Component Amyloid Fibrils: The Role of Coulombic Interactions
- PMID: 37492019
- PMCID: PMC10405213
- DOI: 10.1021/acs.jpcb.3c00976
Self-Assembly of Insulin-Derived Chimeric Peptides into Two-Component Amyloid Fibrils: The Role of Coulombic Interactions
Abstract
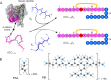
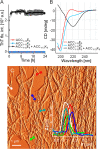
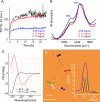
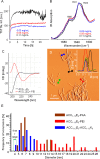
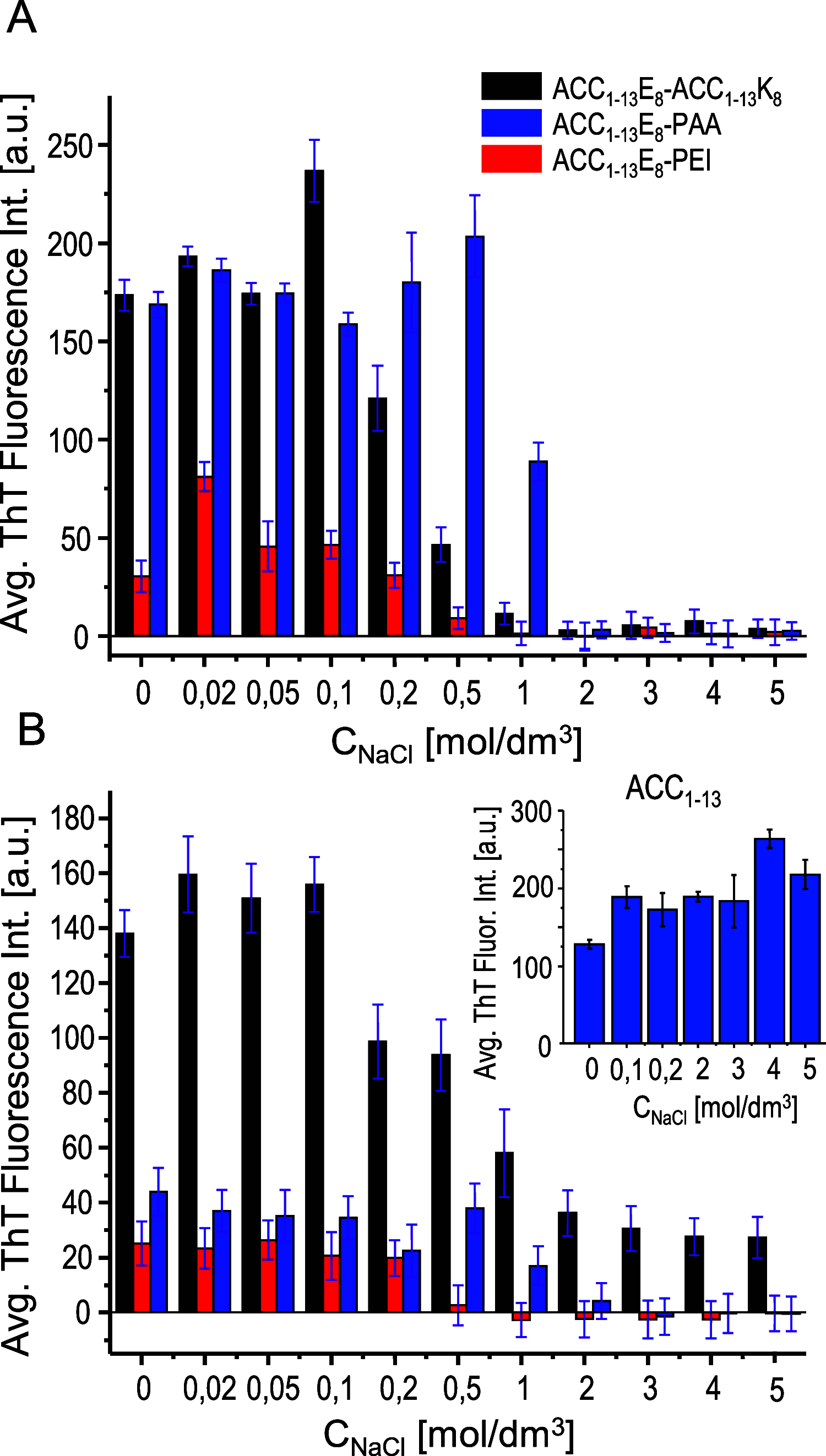
Canonical amyloid fibrils are composed of covalently identical polypeptide chains. Here, we employ kinetic assays, atomic force microscopy, infrared spectroscopy, circular dichroism, and molecular dynamics simulations to study fibrillization patterns of two chimeric peptides, ACC1-13E8 and ACC1-13K8, in which a potent amyloidogenic stretch derived from the N-terminal segment of the insulin A-chain (ACC1-13) is coupled to octaglutamate or octalysine segments, respectively. While large electric charges prevent aggregation of either peptide at neutral pH, stoichiometric mixing of ACC1-13E8 and ACC1-13K8 triggers rapid self-assembly of two-component fibrils driven by favorable Coulombic interactions. The low-symmetry nonpolar ACC1-13 pilot sequence is crucial in enforcing the fibrillar structure consisting of parallel β-sheets as the self-assembly of free poly-E and poly-K chains under similar conditions results in amorphous antiparallel β-sheets. Interestingly, ACC1-13E8 forms highly ordered fibrils also when paired with nonpolypeptide polycationic amines such as branched polyethylenimine, instead of ACC1-13K8. Such synthetic polycations are more effective in triggering the fibrillization of ACC1-13E8 than poly-K (or poly-E in the case of ACC1-13K8). The high conformational flexibility of these polyamines makes up for the apparent mismatch in periodicity of charged groups. The results are discussed in the context of mechanisms of heterogeneous disease-related amyloidogenesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Forced amyloidogenic cooperativity of structurally incompatible peptide segments: Fibrillization behavior of highly aggregation-prone A-chain fragment of insulin coupled to all-L, and alternating L/D octaglutamates.Int J Biol Macromol. 2022 Dec 31;223(Pt A):362-369. doi: 10.1016/j.ijbiomac.2022.11.050. Epub 2022 Nov 9. Int J Biol Macromol. 2022. PMID: 36368353
-
Selective and stoichiometric incorporation of ATP by self-assembling amyloid fibrils.J Mater Chem B. 2021 Oct 27;9(41):8626-8630. doi: 10.1039/d1tb01976g. J Mater Chem B. 2021. PMID: 34622264
-
Liquid-Droplet-Mediated ATP-Triggered Amyloidogenic Pathway of Insulin-Derived Chimeric Peptides: Unraveling the Microscopic and Molecular Processes.J Am Chem Soc. 2023 Feb 10. doi: 10.1021/jacs.2c12611. Online ahead of print. J Am Chem Soc. 2023. PMID: 36762833
-
Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins.Ann N Y Acad Sci. 2005 Dec;1066:181-221. doi: 10.1196/annals.1363.030. Ann N Y Acad Sci. 2005. PMID: 16533927 Review.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Clues to the Design of Aggregation-Resistant Insulin from Proline Scanning of Highly Amyloidogenic Peptides Derived from the N-Terminal Segment of the A-Chain.Mol Pharm. 2024 Apr 1;21(4):2025-2033. doi: 10.1021/acs.molpharmaceut.4c00077. Epub 2024 Mar 25. Mol Pharm. 2024. PMID: 38525800 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

