Potential biomarker of brain response to opioid antagonism in adolescents with eating disorders: a pilot study
- PMID: 37492067
- PMCID: PMC10363723
- DOI: 10.3389/fpsyt.2023.1161032
Potential biomarker of brain response to opioid antagonism in adolescents with eating disorders: a pilot study
Abstract
Background: Eating Disorders (ED) affect up to 5% of youth and are associated with reward system alterations and compulsive behaviors. Naltrexone, an opioid antagonist, is used to treat ED behaviors such as binge eating and/or purging. The presumed mechanism of action is blockade of reward activation; however, not all patients respond, and the optimal dose is unknown. Developing a tool to detect objective drug response in the brain will facilitate drug development and therapeutic optimization. This pilot study evaluated neuroimaging as a pharmacodynamic biomarker of opioid antagonism in adolescents with ED.
Methods: Youth aged 13-21 with binge/purge ED completed functional magnetic resonance imaging (fMRI) pre- and post-oral naltrexone. fMRI detected blood oxygenation-level dependent (BOLD) signal at rest and during two reward probes (monetary incentive delay, MID, and passive food view, PFV) in predefined regions of interest associated with reward and inhibitory control. Effect sizes for Δ%BOLD (post-naltrexone vs. baseline) were estimated using linear mixed effects modeling.
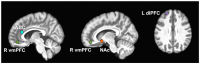
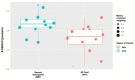
Results: In 12 youth (16-21 years, 92% female), BOLD signal changes were detected following naltrexone in the nucleus accumbens during PFV (Δ%BOLD -0.08 ± 0.03; Cohen's d -1.06, p = 0.048) and anterior cingulate cortex during MID (Δ%BOLD 0.06 ± 0.03; Cohen's d 1.25, p = 0.086).
Conclusion: fMRI detected acute reward pathway modulation in this small sample of adolescents with binge/purge ED. If validated in future, larger trials, task-based Δ%BOLD detected by fMRI may serve as a pharmacodynamic biomarker of opioid antagonism to facilitate the development of novel therapeutics targeting the reward pathway, enable quantitative pharmacology trials, and inform drug dosing.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT04935931, NCT#04935931.
Keywords: adolescents; eating disorders; fMRI; naltrexone; opioid antagonism; pharmacodynamic biomarker.
Copyright © 2023 Stancil, Yeh, Brucks, Bruce, Voss, Abdel-Rahman, Brooks and Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Development of a pharmacodynamic biomarker of opioid antagonism in adolescents with eating disorders: Study protocol for the naltrexone neuroimaging randomized controlled trial (NN-RCT).Contemp Clin Trials. 2025 May;152:107874. doi: 10.1016/j.cct.2025.107874. Epub 2025 Mar 3. Contemp Clin Trials. 2025. PMID: 40043750
-
Opioid receptor antagonism and neural response to monetary rewards: Pilot studies in light and heavy alcohol users.J Psychopharmacol. 2023 Sep;37(9):937-941. doi: 10.1177/02698811231191707. Epub 2023 Aug 2. J Psychopharmacol. 2023. PMID: 37530456 Clinical Trial.
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
-
Naltrexone Reduces Binge Eating and Purging in Adolescents in an Eating Disorder Program.J Child Adolesc Psychopharmacol. 2019 Nov;29(9):721-724. doi: 10.1089/cap.2019.0056. Epub 2019 Jul 16. J Child Adolesc Psychopharmacol. 2019. PMID: 31313939 Free PMC article.
-
Lessons learned from using fMRI in the early clinical development of a mu-opioid receptor antagonist for disorders of compulsive consumption.Psychopharmacology (Berl). 2021 May;238(5):1255-1263. doi: 10.1007/s00213-019-05427-5. Epub 2020 Jan 4. Psychopharmacology (Berl). 2021. PMID: 31900526 Review.
Cited by
-
Development of a pharmacodynamic biomarker of opioid antagonism in adolescents with eating disorders: Study protocol for the naltrexone neuroimaging randomized controlled trial (NN-RCT).Contemp Clin Trials. 2025 May;152:107874. doi: 10.1016/j.cct.2025.107874. Epub 2025 Mar 3. Contemp Clin Trials. 2025. PMID: 40043750
-
Neuroimaging as a Tool for Advancing Pediatric Psychopharmacology.Paediatr Drugs. 2025 May;27(3):307-330. doi: 10.1007/s40272-025-00683-9. Epub 2025 Feb 3. Paediatr Drugs. 2025. PMID: 39899194 Review.
References
-
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. (2011) 68:714–23. doi: 10.1001/archgenpsychiatry.2011.22 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

