Evaluation of chronic drug-induced electrophysiological and cytotoxic effects using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)
- PMID: 37492082
- PMCID: PMC10364322
- DOI: 10.3389/fphar.2023.1229960
Evaluation of chronic drug-induced electrophysiological and cytotoxic effects using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)
Abstract
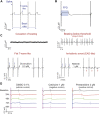
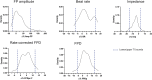
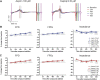
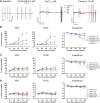
Introduction: Cardiotoxicity is one of the leading causes of compound attrition during drug development. Most in vitro screening platforms aim at detecting acute cardio-electrophysiological changes and drug-induced chronic functional alterations are often not studied in the early stage of drug development. Therefore, we developed an assay using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) that evaluates both drug-induced acute and delayed electrophysiological and cytotoxic effects of reference compounds with clinically known cardiac outcomes. Methods: hiPSC-CMs were seeded in 48-well multielectrode array (MEA) plates and were treated with four doses of reference compounds (covering and exceeding clinical free plasma peak concentrations -fCmax values) and MEA recordings were conducted for 4 days. Functional-electrophysiological (field-potentials) and viability (impedance) parameters were recorded with a MEA machine. Results: To assess this platform, we tested tyrosine-kinase inhibitors with high-cardiac risk profile (sunitinib, vandetanib and nilotinib) and low-cardiac risk (erlotinib), as well as known classic cardiac toxic drugs (doxorubicin and BMS-986094), ion-channel trafficking inhibitors (pentamidine, probucol and arsenic trioxide) and compounds without known clinical cardiotoxicity (amoxicillin, cetirizine, captopril and aspirin). By evaluating the effects of these compounds on MEA parameters, the assay was mostly able to recapitulate different drug-induced cardiotoxicities, represented by a prolongation of the field potential, changes in beating rate and presence of arrhythmic events in acute (<2 h) or delayed phase ≥24 h, and/or reduction of impedance during the delayed phase (≥24 h). Furthermore, a few reference compounds were tested in hiPSC-CMs using fluorescence- and luminescence-based plate reader assays, confirming the presence or absence of cytotoxic effects, linked to changes of the impedance parameters measured in the MEA assay. Of note, some cardiotoxic effects could not be identified at acute time points (<2 h) but were clearly detected after 24 h, reinforcing the importance of chronic drug evaluation. Discussion: In conclusion, the evaluation of chronic drug-induced cardiotoxicity using a hiPSC-CMs in vitro assay can contribute to the early de-risking of compounds and help optimize the drug development process.
Keywords: arrhythmias; cytotoxicity; drug-induced cardiotoxicity; hiPSC-CMs; in vitro assay; multielectrode arrays (MEA); risk prediction.
Copyright © 2023 Altrocchi, Van Ammel, Steemans, Kreir, Tekle, Teisman, Gallacher and Lu.
Conflict of interest statement
Authors CA, KVA, MS, MK, FT, AT, DG, and HRL were employed by Janssen R&D.
Figures


Similar articles
-
Effects of Electrical Stimulation on hiPSC-CM Responses to Classic Ion Channel Blockers.Toxicol Sci. 2020 Apr 1;174(2):254-265. doi: 10.1093/toxsci/kfaa010. Toxicol Sci. 2020. PMID: 32040191
-
Electrophysiological mechanisms of vandetanib-induced cardiotoxicity: Comparison of action potentials in rabbit Purkinje fibers and pluripotent stem cell-derived cardiomyocytes.PLoS One. 2018 Apr 9;13(4):e0195577. doi: 10.1371/journal.pone.0195577. eCollection 2018. PLoS One. 2018. PMID: 29630634 Free PMC article.
-
CSAHi study: Detection of drug-induced ion channel/receptor responses, QT prolongation, and arrhythmia using multi-electrode arrays in combination with human induced pluripotent stem cell-derived cardiomyocytes.J Pharmacol Toxicol Methods. 2017 May-Jun;85:73-81. doi: 10.1016/j.vascn.2017.02.001. Epub 2017 Feb 3. J Pharmacol Toxicol Methods. 2017. PMID: 28163191
-
Cardiotoxicity Assessment of Drugs Using Human iPS Cell-Derived Cardiomyocytes: Toward Proarrhythmic Risk and Cardio-Oncology.Curr Pharm Biotechnol. 2020;21(9):765-772. doi: 10.2174/1389201020666190628143345. Curr Pharm Biotechnol. 2020. PMID: 31264543 Review.
-
Chronic Cardiotoxicity Assays Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs).Int J Mol Sci. 2022 Mar 16;23(6):3199. doi: 10.3390/ijms23063199. Int J Mol Sci. 2022. PMID: 35328619 Free PMC article. Review.
Cited by
-
Cardiovascular Toxicity in Cancer Therapy: Protecting the Heart while Combating Cancer.Curr Cardiol Rep. 2024 Sep;26(9):953-971. doi: 10.1007/s11886-024-02099-2. Epub 2024 Jul 23. Curr Cardiol Rep. 2024. PMID: 39042344 Free PMC article. Review.
-
Exploiting host kinases to combat dengue virus infection and disease.Antiviral Res. 2025 Sep;241:106172. doi: 10.1016/j.antiviral.2025.106172. Epub 2025 May 8. Antiviral Res. 2025. PMID: 40348023 Review.
-
Predicting oncology drug-induced cardiotoxicity with donor-specific iPSC-CMs-a proof-of-concept study with doxorubicin.Toxicol Sci. 2024 Jun 26;200(1):79-94. doi: 10.1093/toxsci/kfae041. Toxicol Sci. 2024. PMID: 38547396 Free PMC article.
-
Editorial: Recent advances in cardiotoxicity testing, volume II.Front Pharmacol. 2024 Apr 29;15:1414373. doi: 10.3389/fphar.2024.1414373. eCollection 2024. Front Pharmacol. 2024. PMID: 38741588 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources

